Liushen Capsules, a promising clinical candidate for COVID-19, alleviates SARS-CoV-2-induced pulmonary in vivo and inhibits the proliferation of the variant virus strains in vitro
- PMID: 35365215
- PMCID: PMC8972667
- DOI: 10.1186/s13020-022-00598-4
Liushen Capsules, a promising clinical candidate for COVID-19, alleviates SARS-CoV-2-induced pulmonary in vivo and inhibits the proliferation of the variant virus strains in vitro
Abstract
Background: Coronavirus disease 2019 (COVID-19) causes a global pandemic and has devastating effects around the world, however, there are no specific antiviral drugs and vaccines for the constant mutation of SARS-CoV-2.
Purpose: In this study, we evaluted the antiviral and anti-inflammatory activities of Liushen Capsules (LS) on different novel coronavirus in vitro, studied its therapeutic effects on novel SARS-CoV-2 infected mice and observed the LS's clinical efficacy and safety in COVID-19.
Methods: The antiviral and aiti-inflammatory effects of LS on the 501Y.V2/B.1.35 and G/478K.V1/ B.1.617.2 strains were determined in vitro. A hACE2 mouse model of novel SARS-CoV-2 pneumonia was established. Survival rates, histological changes, inflammatory markers, lung virus titers and the expression of the key proteins in the NF-κB/MAPK signaling pathway was detected by western blotting and immumohistochemical staining in the lungs were measured. Subsequently, the disease duration, prognosis of disease, time of negative nucleic acid and the cytokines levels in serum were used to assess the efficacy of treatment with LS in patients.
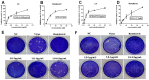
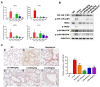
Results: The results showed that LS (2, 1, 0.5 μg/mL) could significantly inhibit the replication of the two SARS-CoV-2 variants and the expression of pro-inflammatory cytokines (IL-6, IL-8, IP-10, CCL-5, MIP-1α, IL-1α) induced by the virus in vitro. As for the survival experiment in mice, the survival rate of virus group was 20%, while LS-treatment groups (40, 80, 160 mg/kg) could increase the survival rate to 60, 100 and 100%, respectively. LS (40, 80, 160 mg/kg) could significantly decrease the lung titers in mice and it could improve the pathological changes, inhibit the excessive inflammatory mediators (IFN-α, IFN-γ, IP-10, MCP-1) and the protein expression of p-NF-κB p65 in mice. Moreover, LS could significantly decrease SARS-CoV-2-induced activation of p-NF-κB p65, p-IκBα, and p-p38 MAPK and increase the protein expression of the IκBα. In addition, the patient got complete relief of symptoms after being treated with LS for 6 days and was proven with negative PCR test after being treated for 23 days. Finally, treatment with LS could reduce the release of inflammatory cytokines (IL-6, PDGF-AA/BB, Eotaxin, MCP-1, MIP-1α, MIP-1β, GRO, CCL-5, MCP-3, IP-10, IL-1α).
Conclusion: LS effectively alleviated novel SARS-CoV-2 or variants induced pneumonia in vitro and in vivo, and improved the prognosis of COVID-19. In light of the efficacy and safety profiles, LS could be considered for the treatment of COVID-19 with a broad-spectrum antiviral and anti-inflammatory agent.
Keywords: COVID-19; Liushen Capsules; Pulmonary; SARS-CoV-2; The 501Y.V2/B.1.35 and G/478K.V1/ B.1.617.2 strains.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interests.
Figures



Similar articles
-
Liu Shen capsule shows antiviral and anti-inflammatory abilities against novel coronavirus SARS-CoV-2 via suppression of NF-κB signaling pathway.Pharmacol Res. 2020 Aug;158:104850. doi: 10.1016/j.phrs.2020.104850. Epub 2020 Apr 30. Pharmacol Res. 2020. PMID: 32360580 Free PMC article.
-
Effect of Jinzhen granule on two coronaviruses: The novel SARS-CoV-2 and the HCoV-229E and the evidences for their mechanisms of action.Phytomedicine. 2022 Jan;95:153874. doi: 10.1016/j.phymed.2021.153874. Epub 2021 Dec 11. Phytomedicine. 2022. PMID: 34923232 Free PMC article.
-
Phillyrin (KD-1) exerts anti-viral and anti-inflammatory activities against novel coronavirus (SARS-CoV-2) and human coronavirus 229E (HCoV-229E) by suppressing the nuclear factor kappa B (NF-κB) signaling pathway.Phytomedicine. 2020 Nov;78:153296. doi: 10.1016/j.phymed.2020.153296. Epub 2020 Aug 1. Phytomedicine. 2020. PMID: 32890913 Free PMC article.
-
Insights into forsythia honeysuckle (Lianhuaqingwen) capsules: A Chinese herbal medicine repurposed for COVID-19 pandemic.Phytomed Plus. 2021 May;1(2):100027. doi: 10.1016/j.phyplu.2021.100027. Epub 2021 Jan 16. Phytomed Plus. 2021. PMID: 35399819 Free PMC article. Review.
-
Disruption of CCR5 signaling to treat COVID-19-associated cytokine storm: Case series of four critically ill patients treated with leronlimab.J Transl Autoimmun. 2021;4:100083. doi: 10.1016/j.jtauto.2021.100083. Epub 2021 Jan 6. J Transl Autoimmun. 2021. PMID: 33521616 Free PMC article. Review.
Cited by
-
Oral Liushen pill for patients with COVID-19: A prospective randomized controlled trial.Pulm Circ. 2023 Jan 1;13(1):e12187. doi: 10.1002/pul2.12187. eCollection 2023 Jan. Pulm Circ. 2023. PMID: 36733313 Free PMC article.
-
Potential antiviral activity of rhamnocitrin against influenza virus H3N2 by inhibiting cGAS/STING pathway in vitro.Sci Rep. 2024 Nov 16;14(1):28287. doi: 10.1038/s41598-024-79788-z. Sci Rep. 2024. PMID: 39550441 Free PMC article.
-
Preclinical evaluation of the SARS-CoV-2 Mpro inhibitor RAY1216 shows improved pharmacokinetics compared with nirmatrelvir.Nat Microbiol. 2024 Apr;9(4):1075-1088. doi: 10.1038/s41564-024-01618-9. Epub 2024 Mar 29. Nat Microbiol. 2024. PMID: 38553607 Free PMC article.
-
Investigating the effects of Laggera pterodonta on H3N2-Induced inflammatory and immune responses through network pharmacology, molecular docking, and experimental validation in a mice model.Heliyon. 2024 Apr 10;10(8):e29487. doi: 10.1016/j.heliyon.2024.e29487. eCollection 2024 Apr 30. Heliyon. 2024. PMID: 38665556 Free PMC article.
-
Investigating the effects of Liushen Capsules on the metabolome of seasonal influenza: A randomized clinical trial.Front Pharmacol. 2022 Aug 11;13:968182. doi: 10.3389/fphar.2022.968182. eCollection 2022. Front Pharmacol. 2022. PMID: 36034844 Free PMC article.
References
-
- Wu ZY, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) Outbreak in China summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. - PubMed
-
- World Health Organization. Tracking SARS-CoV-2 variants. 2021 Accessed 2021.
-
- Wibmer CK, et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. BioRxiv. 2021;586:583. - PubMed
-
- Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383(18):1757–1766. - PubMed
-
- Rochwerg B, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

