Investigation of Neural Substrates of Erroneous Behavior in a Delayed-Response Task
- PMID: 35365501
- PMCID: PMC9007410
- DOI: 10.1523/ENEURO.0490-21.2022
Investigation of Neural Substrates of Erroneous Behavior in a Delayed-Response Task
Abstract
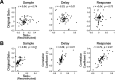
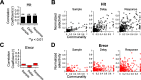
Motor cortical neurons exhibit persistent selective activities (selectivity) during motor planning. Experimental perturbation of selectivity results in the failure of short-term memory retention and consequent behavioral biases, demonstrating selectivity as a neural characteristic of encoding previous sensory input or future action. However, even without experimental manipulation, animals occasionally fail to maintain short-term memory leading to erroneous choice. Here, we investigated neural substrates that lead to the incorrect formation of selectivity during short-term memory. We analyzed neuronal activities in anterior lateral motor cortex (ALM) of mice, a region known to be engaged in motor planning while mice performed the tactile delayed-response task. We found that highly selective neurons lost their selectivity while originally nonselective neurons showed selectivity during the error trials where mice licked toward incorrect direction. We assumed that those alternations would reflect changes in intrinsic properties of population activity. Thus, we estimated an intrinsic manifold shared by neuronal population (shared space), using factor analysis (FA) and measured the association of individual neurons with the shared space by communality, the variance of neuronal activity accounted for by the shared space. We found a positive correlation between selectivity and communality over ALM neurons, which disappeared in erroneous behavior. Notably, neurons showing selectivity alternations between correct and incorrect licking also underwent proportional changes in communality. Our results demonstrated that the extent to which an ALM neuron is associated with the intrinsic manifolds of population activity may elucidate its selectivity and that disruption of this association may alter selectivity, likely leading to erroneous behavior.
Keywords: error behavior; motor planning; premotor cortex; preparatory activity; selectivity; short-term memory.
Copyright © 2022 Chae et al.
Figures

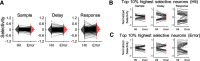





Similar articles
-
Low-Dimensional and Monotonic Preparatory Activity in Mouse Anterior Lateral Motor Cortex.J Neurosci. 2018 Apr 25;38(17):4163-4185. doi: 10.1523/JNEUROSCI.3152-17.2018. Epub 2018 Mar 28. J Neurosci. 2018. PMID: 29593054 Free PMC article.
-
A Map of Anticipatory Activity in Mouse Motor Cortex.Neuron. 2017 May 17;94(4):866-879.e4. doi: 10.1016/j.neuron.2017.05.005. Neuron. 2017. PMID: 28521137
-
Discrete attractor dynamics underlies persistent activity in the frontal cortex.Nature. 2019 Feb;566(7743):212-217. doi: 10.1038/s41586-019-0919-7. Epub 2019 Feb 6. Nature. 2019. PMID: 30728503
-
Control of remembered reaching sequences in monkey. II. Storage and preparation before movement in motor and premotor cortex.Exp Brain Res. 1996 Dec;112(3):347-58. doi: 10.1007/BF00227941. Exp Brain Res. 1996. PMID: 9007537 Review.
-
[Neural correlates of perceptual decisions: the role of the ventral premotor cortex].Rev Neurol. 2014 May 1;58(9):401-10. Rev Neurol. 2014. PMID: 24777768 Review. Spanish.
Cited by
-
Bias in Prestimulus Motor Cortical Activity Determines Decision-making Error in Rodents.Exp Neurobiol. 2023 Aug 31;32(4):271-284. doi: 10.5607/en23020. Exp Neurobiol. 2023. PMID: 37749928 Free PMC article.
-
Distinct hippocampal-prefrontal neural assemblies coordinate memory encoding, maintenance, and recall.Curr Biol. 2023 Apr 10;33(7):1220-1236.e4. doi: 10.1016/j.cub.2023.02.029. Epub 2023 Mar 9. Curr Biol. 2023. PMID: 36898372 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
