Blood eosinophils, fractional exhaled nitric oxide and the risk of asthma attacks in randomised controlled trials: protocol for a systemic review and control arm patient-level meta-analysis for clinical prediction modelling
- PMID: 35365539
- PMCID: PMC8977743
- DOI: 10.1136/bmjopen-2021-058215
Blood eosinophils, fractional exhaled nitric oxide and the risk of asthma attacks in randomised controlled trials: protocol for a systemic review and control arm patient-level meta-analysis for clinical prediction modelling
Abstract
Introduction: The reduction of the risk of asthma attacks is a major goal of guidelines. The fact that type-2 inflammatory biomarkers identify a higher risk, anti-inflammatory responsive phenotype is potentially relevant to this goal. We aim to quantify the relation between blood eosinophils, exhaled nitric oxide (FeNO) and the risk of severe asthma attacks.
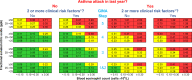
Methods and analysis: A systematic review of randomised controlled trials (RCTs) will be conducted by searching MEDLINE from January 1993 to April 2021. We will include RCTs that investigated the effect of fixed treatment(s) regimen(s) on severe asthma exacerbation rates over at least 24 weeks and reported a baseline value for blood eosinophils and FeNO. Study selection will follow the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, and the methodological appraisal of the studies will be assessed by the Cochrane Risk-of-Bias Tool for RCTs. Study authors will be contacted to request anonymised individual participant data (IPD) for patients randomised to the trial's control arm. An IPD meta-analysis will be performed for multivariable prognostic modelling with performance assessment (calibration plots and the c-statistic) in a cross-validation by study procedure. The outcome to predict is the absolute number of severe asthma attacks to occur in the following 12 months if anti-inflammatory therapy is not changed (ie, annualised number of attacks requiring ≥3 days of systemic corticosteroids and/or hospitalisation if the patient was randomised to the control arm of an RCT). A summary prognostic equation and risk stratification chart will be reported as a basis for further analyses of individualised treatment benefit.
Ethics and dissemination: The protocol has been reviewed by the relevant Oxford academic ethics committee and found to comprise fully anonymised data not requiring further ethical approbation. Results will be communicated in an international meeting and submitted to a peer-reviewed journal.
Prospero registration number: CRD42021245337.
Keywords: asthma; epidemiology; immunology; preventive medicine; thoracic medicine.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SC received a non-restricted research grant from Sanofi-Genzyme for investigator-initiated type 2 innovation research and received speaker honoraria from GlaxoSmithKline, Sanofi-Regeneron and AstraZeneca; outside the submitted work. ES receives royalties from Springer for the textbook entitled Clinical Prediction Models and received speaker honoraria from GlaxoSmithKline; outside the submitted work. RB has received honoraria for presentations or consulting in Adboards from AstraZeneca, Asthma and Respiratory Foundation of New Zealand, Avillion, Cipla and Theravance; and research grants from AstraZeneca, CureKids (NZ), Genentech, and the Health Research Council of New Zealand. IP: in the last 5 years, IP has received speaker’s honoraria for speaking at sponsored meetings from AstraZeneca, Boehringer Ingelheim, Aerocrine AB, Almirall, Novartis, Teva, Chiesi, Sanofi/Regeneron, Menarini, and GSK, and payments for organising educational events from AstraZeneca, GSK, Sanofi/Regeneron, and Teva. He has received honoraria for attending advisory panels with Genentech, Sanofi/Regeneron, AstraZeneca, Boehringer Ingelheim, GSK, Novartis, Teva, Merck, Circassia, Chiesi, and Knopp, and payments to support FDA approval meetings from GSK. He has received sponsorship to attend international scientific meetings from Boehringer Ingelheim, GSK, AstraZeneca, Teva, and Chiesi. He has received a grant from Chiesi to support a phase 2 clinical trial in Oxford. He is copatent holder of the rights to the Leicester Cough Questionnaire and has received payments for its use in clinical trials from Merck, Bayer, and Insmed. In 2014–2015 he was an expert witness for a patent dispute involving AstraZeneca and Teva.
Figures
References
-
- Global Initiative for Asthma (GINA) . Global strategy for asthma management and prevention (2021 update), 2021. Available: https://ginasthma.org/
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
