NFATc1 signaling drives chronic ER stress responses to promote NAFLD progression
- PMID: 35365570
- PMCID: PMC9664107
- DOI: 10.1136/gutjnl-2021-325013
NFATc1 signaling drives chronic ER stress responses to promote NAFLD progression
Abstract
Objectives: Non-alcoholic fatty liver disease (NAFLD) can persist in the stage of simple hepatic steatosis or progress to steatohepatitis (NASH) with an increased risk for cirrhosis and cancer. We examined the mechanisms controlling the progression to severe NASH in order to develop future treatment strategies for this disease.
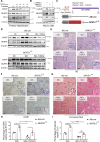
Design: NFATc1 activation and regulation was examined in livers from patients with NAFLD, cultured and primary hepatocytes and in transgenic mice with differential hepatocyte-specific expression of the transcription factor (Alb-cre, NFATc1c.a . and NFATc1Δ/Δ ). Animals were fed with high-fat western diet (WD) alone or in combination with tauroursodeoxycholic acid (TUDCA), a candidate drug for NAFLD treatment. NFATc1-dependent ER stress-responses, NLRP3 inflammasome activation and disease progression were assessed both in vitro and in vivo.
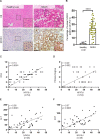
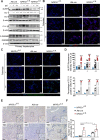
Results: NFATc1 expression was weak in healthy livers but strongly induced in advanced NAFLD stages, where it correlates with liver enzyme values as well as hepatic inflammation and fibrosis. Moreover, high-fat WD increased NFATc1 expression, nuclear localisation and activation to promote NAFLD progression, whereas hepatocyte-specific depletion of the transcription factor can prevent mice from disease acceleration. Mechanistically, NFATc1 drives liver cell damage and inflammation through ER stress sensing and activation of the PERK-CHOP unfolded protein response (UPR). Finally, NFATc1-induced disease progression towards NASH can be blocked by TUDCA administration.
Conclusion: NFATc1 stimulates NAFLD progression through chronic ER stress sensing and subsequent activation of terminal UPR signalling in hepatocytes. Interfering with ER stress-responses, for example, by TUDCA, protects fatty livers from progression towards manifest NASH.
Keywords: fatty liver; hepatic fibrosis; inflammation; nonalcoholic steatohepatitis.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
