Clinical, genomic, and transcriptomic correlates of response to immune checkpoint blockade-based therapy in a cohort of patients with angiosarcoma treated at a single center
- PMID: 35365586
- PMCID: PMC8977792
- DOI: 10.1136/jitc-2021-004149
Clinical, genomic, and transcriptomic correlates of response to immune checkpoint blockade-based therapy in a cohort of patients with angiosarcoma treated at a single center
Abstract
Background: Angiosarcoma is a histologically and molecularly heterogeneous vascular neoplasm with aggressive clinical behavior. Emerging data suggests that immune checkpoint blockade (ICB) is efficacious against some angiosarcomas, particularly cutaneous angiosarcoma of the head and neck (CHN).
Methods: Patients with histologically confirmed angiosarcoma treated with ICB-based therapy at a comprehensive cancer center were retrospectively identified. Clinical characteristics and the results of targeted exome sequencing, transcriptome sequencing, and immunohistochemistry analyses were examined for correlation with clinical benefit. Durable clinical benefit was defined as a progression-free survival (PFS) of ≥16 weeks.
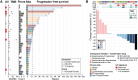
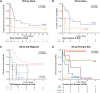
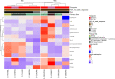
Results: For the 35 patients included in the analyses, median PFS and median overall survival (OS) from the time of first ICB-based treatment were 11.9 (95% CI 7.4 to 31.9) and 42.5 (95% CI 19.6 to 114.2) weeks, respectively. Thirteen patients (37%) had PFS ≥16 weeks. Clinical factors associated with longer PFS and longer OS in multivariate analyses were ICB plus other therapy regimens, CHN disease, and white race. Three of 10 patients with CHN angiosarcoma evaluable for tumor mutational burden (TMB) had a TMB ≥10. Five of six patients with CHN angiosarcoma evaluable for mutational signature analysis had a dominant mutational signature associated with ultraviolet (UV) light. No individual gene or genomic pathway was significantly associated with PFS or OS; neither were TMB or UV signature status. Analyses of whole transcriptomes from nine patient tumor samples found upregulation of angiogenesis, inflammatory response, and KRAS signaling pathways, among others, in patients with PFS ≥16 weeks, as well as higher levels of cytotoxic T cells, dendritic cells, and natural killer cells. Patients with PFS <16 weeks had higher numbers of cancer-associated fibroblasts. Immunohistochemistry findings for 12 patients with baseline samples available suggest that neither PD-L1 expression nor presence of tumor-infiltrating lymphocytes at baseline appears necessary for a response to ICB-based therapy.
Conclusions: ICB-based therapy benefits only a subset of angiosarcoma patients. Patients with CHN angiosarcoma are more likely to have PFS ≥16 weeks, a dominant UV mutational signature, and higher TMB than angiosarcomas arising from other primary sites. However, clinical benefit was seen in other angiosarcomas also and was not restricted to tumors with a high TMB, a dominant UV signature, PD-L1 expression, or presence of tumor infiltrating lymphocytes at baseline.
Keywords: Biomarkers, Tumor; Immunotherapy; Sarcoma.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

Similar articles
-
Association of Survival and Immune-Related Biomarkers With Immunotherapy in Patients With Non-Small Cell Lung Cancer: A Meta-analysis and Individual Patient-Level Analysis.JAMA Netw Open. 2019 Jul 3;2(7):e196879. doi: 10.1001/jamanetworkopen.2019.6879. JAMA Netw Open. 2019. PMID: 31290993 Free PMC article.
-
Oncogene-specific differences in tumor mutational burden, PD-L1 expression, and outcomes from immunotherapy in non-small cell lung cancer.J Immunother Cancer. 2021 Aug;9(8):e002891. doi: 10.1136/jitc-2021-002891. J Immunother Cancer. 2021. PMID: 34376553 Free PMC article.
-
Clinical Implications of Circulating Tumor DNA Tumor Mutational Burden (ctDNA TMB) in Non-Small Cell Lung Cancer.Oncologist. 2019 Jun;24(6):820-828. doi: 10.1634/theoncologist.2018-0433. Epub 2019 Mar 13. Oncologist. 2019. PMID: 30867242 Free PMC article.
-
Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic.Ann Oncol. 2019 Jan 1;30(1):44-56. doi: 10.1093/annonc/mdy495. Ann Oncol. 2019. PMID: 30395155 Free PMC article. Review.
-
Leveraging big data of immune checkpoint blockade response identifies novel potential targets.Ann Oncol. 2022 Dec;33(12):1304-1317. doi: 10.1016/j.annonc.2022.08.084. Epub 2022 Aug 30. Ann Oncol. 2022. PMID: 36055464 Review.
Cited by
-
Genomic Landscape Comparison of Cardiac versus Extra-Cardiac Angiosarcomas.Biomedicines. 2023 Dec 12;11(12):3290. doi: 10.3390/biomedicines11123290. Biomedicines. 2023. PMID: 38137511 Free PMC article.
-
Dissecting the tumor microenvironment in primary breast angiosarcoma: insights from single-cell RNA sequencing.Breast Cancer Res. 2025 Jun 5;27(1):101. doi: 10.1186/s13058-025-02022-9. Breast Cancer Res. 2025. PMID: 40474296 Free PMC article.
-
Primary Breast Angiosarcoma: Comparative Transcriptome Analysis.Int J Mol Sci. 2022 Dec 16;23(24):16032. doi: 10.3390/ijms232416032. Int J Mol Sci. 2022. PMID: 36555675 Free PMC article.
-
Multiomics characterization of breast angiosarcoma from an Asian cohort reveals enrichment for angiogenesis signaling pathway and tumor-infiltrating macrophages.Front Immunol. 2025 Jan 13;15:1515935. doi: 10.3389/fimmu.2024.1515935. eCollection 2024. Front Immunol. 2025. PMID: 39872529 Free PMC article.
-
NK cell based immunotherapy against oral squamous cell carcinoma.Front Immunol. 2024 Aug 13;15:1440764. doi: 10.3389/fimmu.2024.1440764. eCollection 2024. Front Immunol. 2024. PMID: 39192980 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
