Defining the early stages of intestinal colonisation by whipworms
- PMID: 35365634
- PMCID: PMC8976045
- DOI: 10.1038/s41467-022-29334-0
Defining the early stages of intestinal colonisation by whipworms
Abstract
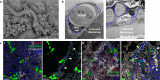
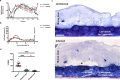
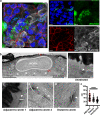
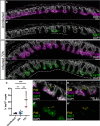
Whipworms are large metazoan parasites that inhabit multi-intracellular epithelial tunnels in the large intestine of their hosts, causing chronic disease in humans and other mammals. How first-stage larvae invade host epithelia and establish infection remains unclear. Here we investigate early infection events using both Trichuris muris infections of mice and murine caecaloids, the first in-vitro system for whipworm infection and organoid model for live helminths. We show that larvae degrade mucus layers to access epithelial cells. In early syncytial tunnels, larvae are completely intracellular, woven through multiple live dividing cells. Using single-cell RNA sequencing of infected mouse caecum, we reveal that progression of infection results in cell damage and an expansion of enterocytes expressing of Isg15, potentially instigating the host immune response to the whipworm and tissue repair. Our results unravel intestinal epithelium invasion by whipworms and reveal specific host-parasite interactions that allow the whipworm to establish its multi-intracellular niche.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Else KJ, et al. Whipworm and roundworm infections. Nat. Rev. Dis. Prim. 2020;6:44. - PubMed
-
- Jourdan PM, Lamberton PHL, Fenwick A, Addiss DG. Soil-transmitted helminth infections. Lancet. 2018;391:252–265. - PubMed
-
- Panesar TS, Croll NA. The location of parasites within their hosts: site selection by Trichuris muris in the laboratory mouse. Int. J. Parasitol. 1980;10:261–273. - PubMed
-
- Tilney LG, Connelly PS, Guild GM, Vranich KA, Artis D. Adaptation of a nematode parasite to living within the mammalian epithelium. J. Exp. Zool. A Comp. Exp. Biol. 2005;303:927–945. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

