A benzodiazepine activator locks Kv7.1 channels open by electro-mechanical uncoupling
- PMID: 35365746
- PMCID: PMC8976019
- DOI: 10.1038/s42003-022-03229-8
A benzodiazepine activator locks Kv7.1 channels open by electro-mechanical uncoupling
Abstract
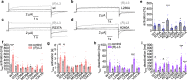
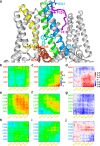
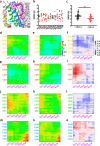
Loss-of-function mutations in Kv7.1 often lead to long QT syndrome (LQTS), a cardiac repolarization disorder associated with arrhythmia and subsequent sudden cardiac death. The discovery of agonistic IKs modulators may offer a new potential strategy in pharmacological treatment of this disorder. The benzodiazepine derivative (R)-L3 potently activates Kv7.1 channels and shortens action potential duration, thus may represent a starting point for drug development. However, the molecular mechanisms underlying modulation by (R)-L3 are still unknown. By combining alanine scanning mutagenesis, non-canonical amino acid incorporation, voltage-clamp electrophysiology and fluorometry, and in silico protein modelling, we show that (R)-L3 not only stimulates currents by allosteric modulation of the pore domain but also alters the kinetics independently from the pore domain effects. We identify novel (R)-L3-interacting key residues in the lower S4-segment of Kv7.1 and observed an uncoupling of the outer S4 segment with the inner S5, S6 and selectivity filter segments.
© 2022. The Author(s).
Conflict of interest statement
The authors declare the following competing interests: J.i.S. and J.C. are cofounders of a startup company VivoCor LLC, which is targeting IKs for the treatment of cardiac arrhythmia. Other authors declare they have no competing interests.
Figures





References
-
- Jespersen T, Grunnet M, Olesen SP. The KCNQ1 potassium channel: From gene to physiological function. Physiology. 2005;20:408–416. - PubMed
-
- Abbott GW, Goldstein SA. A superfamily of small potassium channel subunits: form and function of the MinK-related peptides (MiRPs) Q Rev. Biophys. 1998;31:357–398. - PubMed
-
- McCrossan ZA, Abbott GW. The MinK-related peptides. Neuropharmacology. 2004;47:787–821. - PubMed
-
- Piccini M, et al. KCNE1-like gene is deleted in AMME contiguous gene syndrome: identification and characterization of the human and mouse homologs. Genomics. 1999;60:251–257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

