Viruses affect picocyanobacterial abundance and biogeography in the North Pacific Ocean
- PMID: 35365792
- PMCID: PMC8975747
- DOI: 10.1038/s41564-022-01088-x
Viruses affect picocyanobacterial abundance and biogeography in the North Pacific Ocean
Abstract
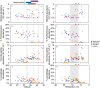
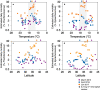
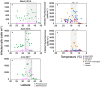
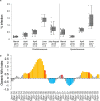
The photosynthetic picocyanobacteria Prochlorococcus and Synechococcus are models for dissecting how ecological niches are defined by environmental conditions, but how interactions with bacteriophages affect picocyanobacterial biogeography in open ocean biomes has rarely been assessed. We applied single-virus and single-cell infection approaches to quantify cyanophage abundance and infected picocyanobacteria in 87 surface water samples from five transects that traversed approximately 2,200 km in the North Pacific Ocean on three cruises, with a duration of 2-4 weeks, between 2015 and 2017. We detected a 550-km-wide hotspot of cyanophages and virus-infected picocyanobacteria in the transition zone between the North Pacific Subtropical and Subpolar gyres that was present in each transect. Notably, the hotspot occurred at a consistent temperature and displayed distinct cyanophage-lineage composition on all transects. On two of these transects, the levels of infection in the hotspot were estimated to be sufficient to substantially limit the geographical range of Prochlorococcus. Coincident with the detection of high levels of virally infected picocyanobacteria, we measured an increase of 10-100-fold in the Synechococcus populations in samples that are usually dominated by Prochlorococcus. We developed a multiple regression model of cyanophages, temperature and chlorophyll concentrations that inferred that the hotspot extended across the North Pacific Ocean, creating a biological boundary between gyres, with the potential to release organic matter comparable to that of the sevenfold-larger North Pacific Subtropical Gyre. Our results highlight the probable impact of viruses on large-scale phytoplankton biogeography and biogeochemistry in distinct regions of the oceans.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
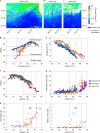
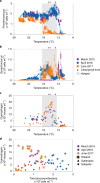
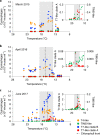
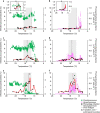






Comment in
-
Life, death and cyanobacterial biogeography.Nat Microbiol. 2022 Apr;7(4):480-481. doi: 10.1038/s41564-022-01095-y. Nat Microbiol. 2022. PMID: 35365793 No abstract available.
Similar articles
-
Genetic Diversity and Cooccurrence Patterns of Marine Cyanopodoviruses and Picocyanobacteria.Appl Environ Microbiol. 2018 Aug 1;84(16):e00591-18. doi: 10.1128/AEM.00591-18. Print 2018 Aug 15. Appl Environ Microbiol. 2018. PMID: 29915108 Free PMC article.
-
Metagenomic Analysis of the Indian Ocean Picocyanobacterial Community: Structure, Potential Function and Evolution.PLoS One. 2016 May 19;11(5):e0155757. doi: 10.1371/journal.pone.0155757. eCollection 2016. PLoS One. 2016. PMID: 27196065 Free PMC article.
-
Cold Surface Waters of the Sub-Antarctic Pacific Ocean Support High Cyanophage Abundances and Infection Levels.Environ Microbiol. 2025 Jan;27(1):e70031. doi: 10.1111/1462-2920.70031. Environ Microbiol. 2025. PMID: 39797436 Free PMC article.
-
Mixotrophy in marine picocyanobacteria: use of organic compounds by Prochlorococcus and Synechococcus.ISME J. 2020 May;14(5):1065-1073. doi: 10.1038/s41396-020-0603-9. Epub 2020 Feb 7. ISME J. 2020. PMID: 32034281 Free PMC article. Review.
-
Prochlorococcus: advantages and limits of minimalism.Ann Rev Mar Sci. 2010;2:305-31. doi: 10.1146/annurev-marine-120308-081034. Ann Rev Mar Sci. 2010. PMID: 21141667 Review.
Cited by
-
Viral infection in the ocean-A journey across scales.PLoS Biol. 2023 Jan 26;21(1):e3001966. doi: 10.1371/journal.pbio.3001966. eCollection 2023 Jan. PLoS Biol. 2023. PMID: 36701270 Free PMC article.
-
Shifts in phytoplankton community structure across oceanic boundaries.PLoS One. 2025 Jun 5;20(6):e0324466. doi: 10.1371/journal.pone.0324466. eCollection 2025. PLoS One. 2025. PMID: 40471890 Free PMC article.
-
Adaptive loss of tRNA gene expression leads to phage resistance in a marine Synechococcus cyanobacterium.Nat Microbiol. 2025 Jan;10(1):66-76. doi: 10.1038/s41564-024-01877-6. Epub 2025 Jan 3. Nat Microbiol. 2025. PMID: 39753669 Free PMC article.
-
A Cyanophage MarR-Type Transcription Factor Regulates Host RNase E Expression during Infection.Microorganisms. 2022 Nov 13;10(11):2245. doi: 10.3390/microorganisms10112245. Microorganisms. 2022. PMID: 36422315 Free PMC article.
-
Assessment of Explicit Representation of Dynamic Viral Processes in Regional Marine Ecological Models.Viruses. 2022 Jun 30;14(7):1448. doi: 10.3390/v14071448. Viruses. 2022. PMID: 35891428 Free PMC article.
References
-
- Moore CM, et al. Processes and patterns of oceanic nutrient limitation. Nat. Geosci. 2013;6:701–710.
-
- Cullen, J., Franks, P., Karl, D. M. & Longhurst, A. in The Sea Vol. 12 (eds Robinson, A. R. et al.) 297–335 (Wiley, 2002).
-
- Thomas MK, Kremer CT, Klausmeier CA, Litchman E. A global pattern of thermal adaptation in marine phytoplankton. Science. 2012;338:1085–1088. - PubMed
-
- Browning TJ, et al. Nutrient co-limitation at the boundary of an oceanic gyre. Nature. 2017;551:242–246. - PubMed
-
- Longhurst, A. R. Ecological Geography of the Sea (Academic Press, 2007).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

