Astrocytes derived from ASD individuals alter behavior and destabilize neuronal activity through aberrant Ca2+ signaling
- PMID: 35365802
- PMCID: PMC9135629
- DOI: 10.1038/s41380-022-01486-x
Astrocytes derived from ASD individuals alter behavior and destabilize neuronal activity through aberrant Ca2+ signaling
Abstract
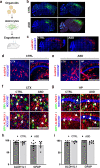
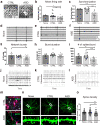
The cellular mechanisms of autism spectrum disorder (ASD) are poorly understood. Cumulative evidence suggests that abnormal synapse function underlies many features of this disease. Astrocytes regulate several key neuronal processes, including the formation of synapses and the modulation of synaptic plasticity. Astrocyte abnormalities have also been identified in the postmortem brain tissue of ASD individuals. However, it remains unclear whether astrocyte pathology plays a mechanistic role in ASD, as opposed to a compensatory response. To address this, we combined stem cell culturing with transplantation techniques to determine disease-specific properties inherent to ASD astrocytes. We demonstrate that ASD astrocytes induce repetitive behavior as well as impair memory and long-term potentiation when transplanted into the healthy mouse brain. These in vivo phenotypes were accompanied by reduced neuronal network activity and spine density caused by ASD astrocytes in hippocampal neurons in vitro. Transplanted ASD astrocytes also exhibit exaggerated Ca2+ fluctuations in chimeric brains. Genetic modulation of evoked Ca2+ responses in ASD astrocytes modulates behavior and neuronal activity deficits. Thus, this study determines that astrocytes derived from ASD iPSCs are sufficient to induce repetitive behavior as well as cognitive deficit, suggesting a previously unrecognized primary role for astrocytes in ASD.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
The Interplay of Astrocytes and Neurons in Autism Spectrum Disorder.Adv Neurobiol. 2024;39:269-284. doi: 10.1007/978-3-031-64839-7_11. Adv Neurobiol. 2024. PMID: 39190079 Review.
-
Modeling the Interplay Between Neurons and Astrocytes in Autism Using Human Induced Pluripotent Stem Cells.Biol Psychiatry. 2018 Apr 1;83(7):569-578. doi: 10.1016/j.biopsych.2017.09.021. Epub 2017 Oct 3. Biol Psychiatry. 2018. PMID: 29129319
-
The role of glia in the dysregulation of neuronal spinogenesis in Ube3a-dependent ASD.Exp Neurol. 2024 Jun;376:114756. doi: 10.1016/j.expneurol.2024.114756. Epub 2024 Mar 18. Exp Neurol. 2024. PMID: 38508482 Free PMC article.
-
NEXMIF/KIDLIA Knock-out Mouse Demonstrates Autism-Like Behaviors, Memory Deficits, and Impairments in Synapse Formation and Function.J Neurosci. 2020 Jan 2;40(1):237-254. doi: 10.1523/JNEUROSCI.0222-19.2019. Epub 2019 Nov 8. J Neurosci. 2020. PMID: 31704787 Free PMC article.
-
Astrocyte plasticity: implications for synaptic and neuronal activity.Neuroscientist. 2013 Dec;19(6):604-15. doi: 10.1177/1073858413504999. Epub 2013 Oct 10. Neuroscientist. 2013. PMID: 24122819 Review.
Cited by
-
The Interplay of Astrocytes and Neurons in Autism Spectrum Disorder.Adv Neurobiol. 2024;39:269-284. doi: 10.1007/978-3-031-64839-7_11. Adv Neurobiol. 2024. PMID: 39190079 Review.
-
Astrocytes: integrators of arousal state and sensory context.Trends Neurosci. 2023 Jun;46(6):418-425. doi: 10.1016/j.tins.2023.03.003. Epub 2023 Mar 30. Trends Neurosci. 2023. PMID: 37003933 Free PMC article. Review.
-
The Synucleins and the Astrocyte.Biology (Basel). 2023 Jan 19;12(2):155. doi: 10.3390/biology12020155. Biology (Basel). 2023. PMID: 36829434 Free PMC article. Review.
-
Simultaneous CRISPR screening and spatial transcriptomics reveal intracellular, intercellular, and functional transcriptional circuits.Cell. 2025 Apr 17;188(8):2141-2158.e18. doi: 10.1016/j.cell.2025.02.012. Epub 2025 Mar 12. Cell. 2025. PMID: 40081369 Free PMC article.
-
Alterations of Purinergic Receptors Levels and Their Involvement in the Glial Cell Morphology in a Pre-Clinical Model of Autism Spectrum Disorders.Brain Sci. 2023 Jul 18;13(7):1088. doi: 10.3390/brainsci13071088. Brain Sci. 2023. PMID: 37509018 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

