Dopamine-induced pruning in monocyte-derived-neuronal-like cells (MDNCs) from patients with schizophrenia
- PMID: 35365810
- PMCID: PMC9156413
- DOI: 10.1038/s41380-022-01514-w
Dopamine-induced pruning in monocyte-derived-neuronal-like cells (MDNCs) from patients with schizophrenia
Abstract
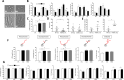
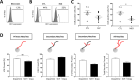
The long lapse between the presumptive origin of schizophrenia (SCZ) during early development and its diagnosis in late adolescence has hindered the study of crucial neurodevelopmental processes directly in living patients. Dopamine, a neurotransmitter consistently associated with the pathophysiology of SCZ, participates in several aspects of brain development including pruning of neuronal extensions. Excessive pruning is considered the cause of the most consistent finding in SCZ, namely decreased brain volume. It is therefore possible that patients with SCZ carry an increased susceptibility to dopamine's pruning effects and that this susceptibility would be more obvious in the early stages of neuronal development when dopamine pruning effects appear to be more prominent. Obtaining developing neurons from living patients is not feasible. Instead, we used Monocyte-Derived-Neuronal-like Cells (MDNCs) as these cells can be generated in only 20 days and deliver reproducible results. In this study, we expanded the number of individuals in whom we tested the reproducibility of MDNCs. We also deepened the characterization of MDNCs by comparing its neurostructure to that of human developing neurons. Moreover, we studied MDNCs from 12 controls and 13 patients with SCZ. Patients' cells differentiate more efficiently, extend longer secondary neurites and grow more primary neurites. In addition, MDNCs from medicated patients expresses less D1R and prune more primary neurites when exposed to dopamine. Haloperidol did not influence our results but the role of other antipsychotics was not examined and thus, needs to be considered as a confounder.
© 2022. The Author(s).
Conflict of interest statement
This protocol is patented in the USA (99932556 (B2)) and Europe (2862926 (A1 & B1)). This patent is held by AB, MOK, VF, TMJ and AH in collaboration with INSERM and SATT IDF-Innov. The authors report no other financial conflict of interest related to this manuscript.
Figures



Similar articles
-
Optimization of Neurite Tracing and Further Characterization of Human Monocyte-Derived-Neuronal-like Cells.Brain Sci. 2021 Oct 20;11(11):1372. doi: 10.3390/brainsci11111372. Brain Sci. 2021. PMID: 34827371 Free PMC article.
-
Transdifferentiation of Human Circulating Monocytes Into Neuronal-Like Cells in 20 Days and Without Reprograming.Front Mol Neurosci. 2018 Sep 19;11:323. doi: 10.3389/fnmol.2018.00323. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30760979 Free PMC article.
-
Abnormal synaptic pruning during adolescence underlying the development of psychotic disorders.Curr Opin Psychiatry. 2021 May 1;34(3):222-227. doi: 10.1097/YCO.0000000000000696. Curr Opin Psychiatry. 2021. PMID: 33560023 Free PMC article. Review.
-
Impact of nuclear distribution element genes in the typical and atypical antipsychotics effects on nematode Caenorhabditis elegans: Putative animal model for studying the pathways correlated to schizophrenia.Prog Neuropsychopharmacol Biol Psychiatry. 2019 Jun 8;92:19-30. doi: 10.1016/j.pnpbp.2018.12.010. Epub 2018 Dec 19. Prog Neuropsychopharmacol Biol Psychiatry. 2019. PMID: 30578843
-
Microglia Activation and Schizophrenia: Lessons From the Effects of Minocycline on Postnatal Neurogenesis, Neuronal Survival and Synaptic Pruning.Schizophr Bull. 2017 May 1;43(3):493-496. doi: 10.1093/schbul/sbw088. Schizophr Bull. 2017. PMID: 27352782 Free PMC article. Review.
Cited by
-
Advances in the role of the GADD45 family in neurodevelopmental, neurodegenerative, and neuropsychiatric disorders.Front Neurosci. 2024 Jan 25;18:1349409. doi: 10.3389/fnins.2024.1349409. eCollection 2024. Front Neurosci. 2024. PMID: 38332860 Free PMC article. Review.
-
Bipolar disorders and schizophrenia: discrete disorders?Front Psychiatry. 2024 Apr 30;15:1352250. doi: 10.3389/fpsyt.2024.1352250. eCollection 2024. Front Psychiatry. 2024. PMID: 38745778 Free PMC article. Review.
-
Dopaminergic Perturbation in the Aetiology of Neurodevelopmental Disorders.Mol Neurobiol. 2025 Feb;62(2):2420-2434. doi: 10.1007/s12035-024-04418-8. Epub 2024 Aug 7. Mol Neurobiol. 2025. PMID: 39110391 Review.
-
mTORC1 activation in presumed classical monocytes: observed correlation with human size variation and neuropsychiatric disease.Aging (Albany NY). 2024 Jul 26;16(14):11134-11150. doi: 10.18632/aging.206033. Epub 2024 Jul 26. Aging (Albany NY). 2024. PMID: 39068671 Free PMC article.
-
Hypermethylation and increased expression of the DRD2 gene in schizophrenia.BMC Psychiatry. 2025 Jul 15;25(1):702. doi: 10.1186/s12888-025-07154-y. BMC Psychiatry. 2025. PMID: 40665271 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

