Patterns of amygdala region pathology in LATE-NC: subtypes that differ with regard to TDP-43 histopathology, genetic risk factors, and comorbid pathologies
- PMID: 35366087
- PMCID: PMC9038848
- DOI: 10.1007/s00401-022-02416-5
Patterns of amygdala region pathology in LATE-NC: subtypes that differ with regard to TDP-43 histopathology, genetic risk factors, and comorbid pathologies
Abstract
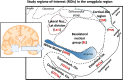
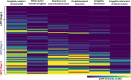
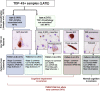
Transactive response (TAR) DNA-binding protein 43 kDa (TDP-43) pathology is a hallmark of limbic-predominant age-related TDP-43 encephalopathy (LATE). The amygdala is affected early in the evolution of LATE neuropathologic change (LATE-NC), and heterogeneity of LATE-NC in amygdala has previously been observed. However, much remains to be learned about how LATE-NC originates and progresses in the brain. To address this, we assessed TDP-43 and other pathologies in the amygdala region of 184 autopsied subjects (median age = 85 years), blinded to clinical diagnoses, other neuropathologic diagnoses, and risk genotype information. As previously described, LATE-NC was associated with older age at death, cognitive impairment, and the TMEM106B risk allele. Pathologically, LATE-NC was associated with comorbid hippocampal sclerosis (HS), myelin loss, and vascular disease in white matter (WM). Unbiased hierarchical clustering of TDP-43 inclusion morphologies revealed discernable subtypes of LATE-NC with distinct clinical, genetic, and pathologic associations. The most common patterns were: Pattern 1, with lamina II TDP-43 + processes and preinclusion pathology in cortices of the amygdala region, and frequent LATE-NC Stage 3 with HS; Pattern 2, previously described as type-β, with neurofibrillary tangle-like TDP-43 neuronal cytoplasmic inclusions (NCIs), high Alzheimer's disease neuropathologic change (ADNC), frequent APOE ε4, and usually LATE-NC Stage 2; Pattern 3, with round NCIs and thick neurites in amygdala, younger age at death, and often comorbid Lewy body disease; and Pattern 4 (the most common pattern), with tortuous TDP-43 processes in subpial and WM regions, low ADNC, rare HS, and lower dementia probability. TDP-43 pathology with features of patterns 1 and 2 were often comorbid in the same brains. Early and mild TDP-43 pathology was often best described to be localized in the "amygdala region" rather than the amygdala proper. There were also important shared attributes across patterns. For example, all four patterns were associated with the TMEM106B risk allele. Each pattern also demonstrated the potential to progress to higher LATE-NC stages with confluent anatomical and pathological patterns, and to contribute to dementia. Although LATE-NC showed distinct patterns of initiation in amygdala region, there was also apparent shared genetic risk and convergent pathways of clinico-pathological evolution.
Keywords: DLB; GRN; Lewy; Neuropathology; Nondemented; PART; Preclinical; Tauopathy.
© 2022. The Author(s).
Conflict of interest statement
The authors report no competing interests.
Figures


Similar articles
-
Limbic-Predominant Age-Related TDP-43 Encephalopathy: Medical and Pathologic Factors Associated With Comorbid Hippocampal Sclerosis.Neurology. 2022 Apr 5;98(14):e1422-e1433. doi: 10.1212/WNL.0000000000200001. Epub 2022 Feb 4. Neurology. 2022. PMID: 35121671 Free PMC article.
-
Cognitive symptoms progress with limbic-predominant age-related TDP-43 encephalopathy stage and co-occurrence with Alzheimer disease.J Neuropathol Exp Neurol. 2023 Dec 22;83(1):2-10. doi: 10.1093/jnen/nlad098. J Neuropathol Exp Neurol. 2023. PMID: 37966908 Free PMC article.
-
Evaluating the updated LATE-NC staging criteria using data from NACC.Alzheimers Dement. 2024 Dec;20(12):8359-8373. doi: 10.1002/alz.14262. Epub 2024 Oct 1. Alzheimers Dement. 2024. PMID: 39352226 Free PMC article.
-
Limbic-predominant age-related TDP-43 encephalopathy (LATE-NC): Co-pathologies and genetic risk factors provide clues about pathogenesis.J Neuropathol Exp Neurol. 2024 May 22;83(6):396-415. doi: 10.1093/jnen/nlae032. J Neuropathol Exp Neurol. 2024. PMID: 38613823 Free PMC article. Review.
-
Transactive response DNA-binding protein 43 (TDP-43) proteinopathy: the complex biological and clinical findings in limbic-predominant age-related TDP-43 encephalopathy (LATE) neuropathological changes, limbic-predominant amnestic neurodegenerative syndrome, and other mixed age-related major neurocognitive disorders.Curr Opin Psychiatry. 2025 Sep 1;38(5):341-347. doi: 10.1097/YCO.0000000000001025. Epub 2025 Jul 16. Curr Opin Psychiatry. 2025. PMID: 40709649 Review.
Cited by
-
Deterioration of neuroimmune homeostasis in Alzheimer's Disease patients who survive a COVID-19 infection.J Neuroinflammation. 2024 Aug 17;21(1):202. doi: 10.1186/s12974-024-03196-3. J Neuroinflammation. 2024. PMID: 39154174 Free PMC article.
-
Old age amyotrophic lateral sclerosis and limbic TDP-43 pathology.Brain Pathol. 2022 Nov;32(6):e13100. doi: 10.1111/bpa.13100. Epub 2022 Jun 17. Brain Pathol. 2022. PMID: 35715944 Free PMC article.
-
TMEM106B Puncta Is Increased in Multiple Sclerosis Plaques, and Reduced Protein in Mice Results in Delayed Lipid Clearance Following CNS Injury.Cells. 2023 Jun 27;12(13):1734. doi: 10.3390/cells12131734. Cells. 2023. PMID: 37443768 Free PMC article.
-
Loss of TDP-43 splicing repression occurs early in the aging population and is associated with Alzheimer's disease neuropathologic changes and cognitive decline.Acta Neuropathol. 2023 Dec 22;147(1):4. doi: 10.1007/s00401-023-02653-2. Acta Neuropathol. 2023. PMID: 38133681
-
Cognitive and Neuropsychological Profiles in Alzheimer's Disease and Primary Age-Related Tauopathy and the Influence of Comorbid Neuropathologies.J Alzheimers Dis. 2023;92(3):1037-1049. doi: 10.3233/JAD-230022. J Alzheimers Dis. 2023. PMID: 36847012 Free PMC article.
References
-
- Agrawal S, Yu L, Nag S, Arfanakis K, Barnes LL, Bennett DA, et al. The association of Lewy bodies with limbic-predominant age-related TDP-43 encephalopathy neuropathologic changes and their role in cognition and Alzheimer's dementia in older persons. Acta Neuropathol Commun. 2021;9:156. doi: 10.1186/s40478-021-01260-0. - DOI - PMC - PubMed
-
- Alheid GF, de Olmos JS, Beltramino CA. Amygdala and extended amygdala. In: Paxinos G, editor. The rat nervous system. 2. London: Academic Press; 1995. pp. 495–578.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

