Hypoxic pretreatment of adipose-derived stem cell exosomes improved cognition by delivery of circ-Epc1 and shifting microglial M1/M2 polarization in an Alzheimer's disease mice model
- PMID: 35366241
- PMCID: PMC9037259
- DOI: 10.18632/aging.203989
Hypoxic pretreatment of adipose-derived stem cell exosomes improved cognition by delivery of circ-Epc1 and shifting microglial M1/M2 polarization in an Alzheimer's disease mice model
Abstract
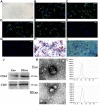
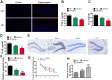
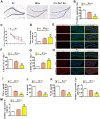
Alzheimer's disease (AD) is the most common dementia in the world. Increasing evidence has shown that exosomes from hypoxic pretreated adipose-derived stem cells (ADSCs) could be an effective cognitive function therapeutic in AD-associated pathophysiology. However, their role and regulatory mechanism remain largely unknown. High-throughput sequencing was used to identify differentially expressed circRNAs from ADSCs or hypoxia pretreated ADSC exosomes. Luciferase reporter assays and RT-qPCR were used to investigate the relationships between circ-Epc1, miR-770-3p, and TREM2. APP/PS1 double transgenic AD model mice were then used to study therapeutic effects regarding circ-Epc1 in ADSC exosomes. BV2 cells were used to show the regulatory relationships between circ-Epc1, miR-770-3p, and TREM2 and to show how these interactions modulated phenotypic transformations and inflammatory cytokine expressions in microglia. The results showed that exosomes from hypoxia pretreated ADSCs had a good therapeutic effect on improving cognitive functions by decreasing neuronal damage in the hippocampus. High-throughput sequencing showed that circ-Epc1 played an important role in hypoxia-pretreated ADSC exosomes regarding their ability to improve cognitive functions. Luciferase reporter assays showed that TREM2 and miR-770-3p were downstream targets of circ-Epc1. Overexpressing miR-770-3p or downregulating TREM2 reversed the effects of circ-Epc1 on M2 microglia during lipopolysaccharide treatment. In vivo experiments showed that circ-Epc1-containing ADSC exosomes increased the therapeutic effect of exosomes by improving cognitive function, decreasing neuronal damage, and shifting hippocampal microglia from the M1 polarization to the M2 polarization stages. Taken together, the data show that hypoxic pretreatment of ADSC exosomes improved cognition by delivery of circ-Epc1 and by shifting microglial M1/M2 polarization in an AD mouse model.
Keywords: Alzheimer's disease; adipose derived stem cells; circ-Epc1; exosomes; microglia.
Conflict of interest statement
Figures



Similar articles
-
Exosomes from hypoxic pre-treated ADSCs attenuate acute ischemic stroke-induced brain injury via delivery of circ-Rps5 and promote M2 microglia/macrophage polarization.Neurosci Lett. 2022 Jan 19;769:136389. doi: 10.1016/j.neulet.2021.136389. Epub 2021 Dec 8. Neurosci Lett. 2022. PMID: 34896256
-
Exosomes from ADSCs ameliorate nerve damage in the hippocampus caused by post traumatic brain injury via the delivery of circ-Scmh1 promoting microglial M2 polarization.Injury. 2023 Oct;54(10):110927. doi: 10.1016/j.injury.2023.110927. Epub 2023 Jul 4. Injury. 2023. PMID: 37544863
-
Exosomes from circRNA-Ptpn4 can modify ADSC treatment and repair nerve damage caused by cerebral infarction by shifting microglial M1/M2 polarization.Mol Cell Biochem. 2024 Aug;479(8):2081-2092. doi: 10.1007/s11010-023-04824-x. Epub 2023 Aug 26. Mol Cell Biochem. 2024. PMID: 37632638
-
TREM2, microglia, and Alzheimer's disease.Mech Ageing Dev. 2021 Apr;195:111438. doi: 10.1016/j.mad.2021.111438. Epub 2021 Jan 28. Mech Ageing Dev. 2021. PMID: 33516818 Review.
-
TREM2 Function in Alzheimer's Disease and Neurodegeneration.ACS Chem Neurosci. 2016 Apr 20;7(4):420-7. doi: 10.1021/acschemneuro.5b00313. Epub 2016 Feb 19. ACS Chem Neurosci. 2016. PMID: 26854967 Review.
Cited by
-
Therapeutic potential of exosomes from adipose-derived stem cells in chronic wound healing.Front Surg. 2022 Sep 30;9:1030288. doi: 10.3389/fsurg.2022.1030288. eCollection 2022. Front Surg. 2022. PMID: 36248361 Free PMC article. Review.
-
Exosomes derived from hypoxia-preconditioned mesenchymal stem cells (hypoMSCs-Exo): advantages in disease treatment.Cell Tissue Res. 2023 Jun;392(3):621-629. doi: 10.1007/s00441-023-03758-6. Epub 2023 Feb 13. Cell Tissue Res. 2023. PMID: 36781483 Review.
-
Exosomes in brain diseases: Pathogenesis and therapeutic targets.MedComm (2020). 2023 Jun 11;4(3):e287. doi: 10.1002/mco2.287. eCollection 2023 Jun. MedComm (2020). 2023. PMID: 37313330 Free PMC article. Review.
-
Exosomes in neurodegenerative diseases: Therapeutic potential and modification methods.Neural Regen Res. 2026 Feb 1;21(2):478-490. doi: 10.4103/NRR.NRR-D-24-00720. Epub 2024 Oct 22. Neural Regen Res. 2026. PMID: 40326981 Free PMC article.
-
Stem cell therapy in Alzheimer's disease: current status and perspectives.Front Neurosci. 2024 Nov 21;18:1440334. doi: 10.3389/fnins.2024.1440334. eCollection 2024. Front Neurosci. 2024. PMID: 39640295 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

