The Impact of CB1 Receptor on Nuclear Receptors in Skeletal Muscle Cells
- PMID: 35366244
- PMCID: PMC8830471
- DOI: 10.3390/pathophysiology28040029
The Impact of CB1 Receptor on Nuclear Receptors in Skeletal Muscle Cells
Abstract
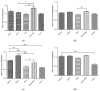
Cannabinoids are abundant signaling compounds; their influence predominantly arises via engagement with the principal two G-protein-coupled cannabinoid receptors, CB1 and CB2. One suggested theory is that cannabinoids regulate a variety of physiological processes within the cells of skeletal muscle. Earlier publications have indicated that expression of CB1 receptor mRNA and protein has been recognized within myotubes and tissues of skeletal muscle from both murines and humans, thus representing a potentially significant pathway which plays a role in the control of skeletal muscular activities. The part played by CB1 receptor activation or inhibition with respect to these functions and relevant to targets in the periphery, especially skeletal muscle, is not fully delineated. Thus, the aim of the current research was to explore the influence of CB1 receptor stimulation and inhibition on downstream signaling of the nuclear receptor, NR4A, which regulates the immediate impacts of arachidonyl-2'-chloroethylamide (ACEA) and/or rimonabant in the cells of skeletal muscle. Murine L6 skeletal muscle cells were used in order to clarify additional possible molecular signaling pathways which contribute to alterations in the CB1 receptor. Skeletal muscle cells have often been used; it is well-documented that they express cannabinoid receptors. Quantitative real-time probe-based polymerase chain reaction (qRT-PCR) assays are deployed in order to assess the gene expression characteristics of CB1 receptor signaling. In the current work, it is demonstrated that skeletal muscle cells exhibit functional expression of CB1 receptors. This can be deduced from the qRT-PCR assays; triggering CB1 receptors amplifies both NR4A1 and NR4A3 mRNA gene expression. The impact of ACEA is inhibited by the selective CB1 receptor antagonist, rimonabant. The present research demonstrated that 10 nM of ACEA notably amplified mRNA gene expression of NR4A1 and NR4A3; this effect was suppressed by the addition of 100 nM rimonabant. Furthermore, the CB1 receptor antagonist led to the downregulation of mRNA gene expression of NR4A1, NR4A2 and NR4A3. In conclusion, in skeletal muscle, CB1 receptors were recognized to be important moderators of NR4A1 and NR4A3 mRNA gene expression; these actions may have possible clinical benefits. Thus, in skeletal muscle cells, a possible physiological expression of CB1 receptors was identified. It is as yet unknown whether these CB1 receptors contribute to pathways underlying skeletal muscle biological function and disease processes. Further research is required to fully delineate their role(s).
Keywords: ACEA; NR4A; cannabinoid CB1 receptors; rimonabant; skeletal muscle cells.
Conflict of interest statement
The author declares that has no conflict of interest.
Figures




Similar articles
-
The Impact of CB1 Receptor on Inflammation in Skeletal Muscle Cells.J Inflamm Res. 2021 Aug 14;14:3959-3967. doi: 10.2147/JIR.S322247. eCollection 2021. J Inflamm Res. 2021. PMID: 34421307 Free PMC article.
-
Inhibitory effects of CB1 and CB2 receptor agonists on responses of DRG neurons and dorsal horn neurons in neuropathic rats.Eur J Neurosci. 2005 Jul;22(2):371-9. doi: 10.1111/j.1460-9568.2005.04206.x. Eur J Neurosci. 2005. PMID: 16045490
-
Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling.J Neurosci. 2002 Nov 15;22(22):9742-53. doi: 10.1523/JNEUROSCI.22-22-09742.2002. J Neurosci. 2002. PMID: 12427829 Free PMC article.
-
The nuclear orphan receptor NR4A1 and NR4A3 as tumor suppressors in hematologic neoplasms.Curr Drug Targets. 2015;16(1):38-46. doi: 10.2174/1389450115666141120112818. Curr Drug Targets. 2015. PMID: 25410408 Review.
-
Pharmacological actions of cannabinoids.Handb Exp Pharmacol. 2005;(168):1-51. doi: 10.1007/3-540-26573-2_1. Handb Exp Pharmacol. 2005. PMID: 16596770 Review.
Cited by
-
Lateral Flow Test System to Control Total Content of Muscle Tissues in Raw Meat Products.Sensors (Basel). 2022 Dec 12;22(24):9724. doi: 10.3390/s22249724. Sensors (Basel). 2022. PMID: 36560100 Free PMC article.
-
Sciatic Nerve Block Combined with Flurbiprofen Inhibits Spinal Cord Inflammation and Improves Postoperative Pain in Rats with Plantar Incision.J Pain Res. 2023 May 9;16:1533-1546. doi: 10.2147/JPR.S404226. eCollection 2023. J Pain Res. 2023. PMID: 37193359 Free PMC article.
-
Comparing the long non-coding RNA expression profiles of skeletal muscle and kidney tissues from patients with diabetes.PLoS One. 2022 Sep 26;17(9):e0274794. doi: 10.1371/journal.pone.0274794. eCollection 2022. PLoS One. 2022. PMID: 36155986 Free PMC article.
-
WIN55212-2 Modulates Intracellular Calcium via CB1 Receptor-Dependent and Independent Mechanisms in Neuroblastoma Cells.Cells. 2022 Sep 21;11(19):2947. doi: 10.3390/cells11192947. Cells. 2022. PMID: 36230909 Free PMC article.
-
Gene Set Enrichment Analysis Detected Immune Cell-Related Pathways Associated with Primary Sclerosing Cholangitis.Biomed Res Int. 2022 Aug 26;2022:2371347. doi: 10.1155/2022/2371347. eCollection 2022. Biomed Res Int. 2022. PMID: 36060137 Free PMC article.
References
-
- Bielawiec P., Harasim-Symbor E., Konstantynowicz-Nowicka K., Sztolsztener K., Chabowski A. Chronic cannabidiol administration attenuates skeletal muscle de novo ceramide synthesis pathway and related metabolic effects in a rat model of high-fat diet-induced obesity. Biomolecules. 2020;10:1241. doi: 10.3390/biom10091241. - DOI - PMC - PubMed
-
- Simcocks A.C., O’Keefe L., Jenkin K.A., Cornall L.M., Grinfeld E., Mathai M.L., Hryciw D.H., McAinch A.J. The Role of Atypical Cannabinoid Ligands O-1602 and O-1918 on Skeletal Muscle Homeostasis with a Focus on Obesity. Int. J. Mol. Sci. 2020;21:5922. doi: 10.3390/ijms21165922. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
