Pathogenesis of Autoimmune Male Infertility: Juxtacrine, Paracrine, and Endocrine Dysregulation
- PMID: 35366245
- PMCID: PMC8830464
- DOI: 10.3390/pathophysiology28040030
Pathogenesis of Autoimmune Male Infertility: Juxtacrine, Paracrine, and Endocrine Dysregulation
Abstract
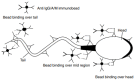
According to global data, there is a male reproductive potential decrease. Pathogenesis of male infertility is often associated with autoimmunity towards sperm antigens essential for fertilization. Antisperm autoantibodies (ASAs) have immobilizing and cytotoxic properties, impairing spermatogenesis, causing sperm agglutination, altering spermatozoa motility and acrosomal reaction, and thus preventing ovum fertilization. Infertility diagnosis requires a mandatory check for the ASAs. The concept of the blood-testis barrier is currently re-formulated, with an emphasis on informational paracrine and juxtacrine effects, rather than simple anatomical separation. The etiology of male infertility includes both autoimmune and non-autoimmune diseases but equally develops through autoimmune links of pathogenesis. Varicocele commonly leads to infertility due to testicular ischemic damage, venous stasis, local hyperthermia, and hypoandrogenism. However, varicocelectomy can alter the blood-testis barrier, facilitating ASAs production as well. There are contradictory data on the role of ASAs in the pathogenesis of varicocele-related infertility. Infection and inflammation both promote ASAs production due to "danger concept" mechanisms and because of antigen mimicry. Systemic pro-autoimmune influences like hyperprolactinemia, hypoandrogenism, and hypothyroidism also facilitate ASAs production. The diagnostic value of various ASAs has not yet been clearly attributed, and their cut-levels have not been determined in sera nor in ejaculate. The assessment of the autoimmunity role in the pathogenesis of male infertility is ambiguous, so the purpose of this review is to show the effects of ASAs on the pathogenesis of male infertility.
Keywords: antisperm autoantibodies; autoimmune thyroiditis; ejaculate; male infertility; orchitis; sperm antigens; spermatozoa; varicocele; varicocelectomy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Xu Y.C., Jing L.I., Liang W.B., Zhu W.J. Evaluation on antisperm antibody in infertile men with oligoasthenoteratozoospermia. J. Reprod. Contracept. 2014;25:49–53. doi: 10.7669/j.issn.1001-7844.2014.01.0049. - DOI
-
- Shevyrin A.A. Modern view on treatment of male infertility. Russ. Med Rev. 2018;12:30–35. (In Russian)
-
- Kalinichenko S.Y., Tyuzikov I.A. Oxidative stress and male infertility are interrelated pandemics of the 21st century. Modern pharmacotherapeutic possibilities of pathogenetic correction of spermatogenesis disorders with L-carnitine/acetyl-L-carnitine preparations. Urol. Nephrol. Spec. Issue Men’s Health. 2017;22:6–19. (In Russian)
-
- Baskaran S., Agarwal A., Selvam M.K.P., Finelli R., Robert K.A., Iovine C., Pushparaj P.N., Samanta L., Harlev A., Henkel R. Tracking Research Trends and Hotspots in Sperm DNA Fragmentation Testing for the Evaluation of Male Infertility: A Scientometric Analysis. Reprod. Biol. Endocrinol. 2019;17:110. doi: 10.1186/s12958-019-0550-3. - DOI - PMC - PubMed
-
- Vybornov S.V., Asfandiyarov F.R., Seidov K.S., Kruglov V.A. Antioxidants in the treatment of patients with inflammatory diseases of the male reproductive system, complicated by excretory toxic form of infertility. Exp. Clin. Urol. 2018;3:74–78. (In Russian)
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
