The Role of Exposomes in the Pathophysiology of Autoimmune Diseases I: Toxic Chemicals and Food
- PMID: 35366249
- PMCID: PMC8830458
- DOI: 10.3390/pathophysiology28040034
The Role of Exposomes in the Pathophysiology of Autoimmune Diseases I: Toxic Chemicals and Food
Abstract
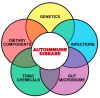
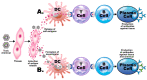
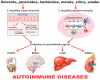
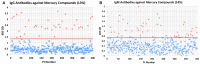
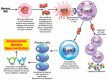
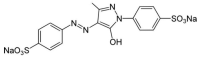
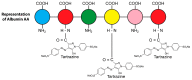
Autoimmune diseases affect 5-9% of the world's population. It is now known that genetics play a relatively small part in the pathophysiology of autoimmune disorders in general, and that environmental factors have a greater role. In this review, we examine the role of the exposome, an individual's lifetime exposure to external and internal factors, in the pathophysiology of autoimmune diseases. The most common of these environmental factors are toxic chemicals, food/diet, and infections. Toxic chemicals are in our food, drink, common products, the air, and even the land we walk on. Toxic chemicals can directly damage self-tissue and cause the release of autoantigens, or can bind to human tissue antigens and form neoantigens, which can provoke autoimmune response leading to autoimmunity. Other types of autoimmune responses can also be induced by toxic chemicals through various effects at the cellular and biochemical levels. The food we eat every day commonly has colorants, preservatives, or packaging-related chemical contamination. The food itself may be antigenic for susceptible individuals. The most common mechanism for food-related autoimmunity is molecular mimicry, in which the food's molecular structure bears a similarity with the structure of one or more self-tissues. The solution is to detect the trigger, remove it from the environment or diet, then repair the damage to the individual's body and health.
Keywords: autoimmune disease; environmental factors; exposome; food; molecular mimicry; toxic chemicals.
Conflict of interest statement
A.V. is co-owner, CEO, and technical director of Immunosciences Lab., Inc. E.V. is owner of Regenera Medical.
Figures







References
Publication types
LinkOut - more resources
Full Text Sources
