Implication of RAS in Postnatal Cardiac Remodeling, Fibrosis and Dysfunction Induced by Fetal Undernutrition
- PMID: 35366262
- PMCID: PMC8830479
- DOI: 10.3390/pathophysiology28020018
Implication of RAS in Postnatal Cardiac Remodeling, Fibrosis and Dysfunction Induced by Fetal Undernutrition
Abstract
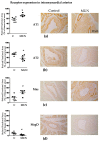
Fetal undernutrition is a risk factor for cardiovascular diseases. Male offspring from rats exposed to undernutrition during gestation (MUN) exhibit oxidative stress during perinatal life and develop cardiac dysfunction in ageing. Angiotensin-II is implicated in oxidative stress-mediated cardiovascular fibrosis and remodeling, and lactation is a key developmental window. We aimed to assess if alterations in RAS during lactation participate in cardiac dysfunction associated with fetal undernutrition. Control dams received food ad libitum, and MUN had 50% nutrient restriction during the second half of gestation. Both dams were fed ad libitum during lactation, and male offspring were studied at weaning. We assessed: ventricular structure and function (echocardiography); blood pressure (intra-arterially, anesthetized rats); collagen content and intramyocardial artery structure (Sirius red, Masson Trichromic); myocardial and intramyocardial artery RAS receptors (immunohistochemistry); plasma angiotensin-II (ELISA) and TGF-β1 protein expression (Western Blot). Compared to Control, MUN offspring exhibited significantly higher plasma Angiotensin-II and a larger left ventricular mass, as well as larger intramyocardial artery media/lumen, interstitial collagen and perivascular collagen. In MUN hearts, TGF-β1 tended to be higher, and the end-diastolic diameter and E/A ratio were significantly lower with no differences in ejection fraction or blood pressure. In the myocardium, no differences between groups were detected in AT1, AT2 or Mas receptors, with MrgD being significantly lower in the MUN group. In intramyocardial arteries from MUN rats, AT1 and Mas receptors were significantly elevated, while AT2 and MrgD were lower compared to Control. Conclusions. In rats exposed to fetal undernutrition, RAS disbalance and associated cardiac remodeling during lactation may set the basis for later heart dysfunction.
Keywords: RAS receptors; angiotensin II; cardiovascular remodeling; fetal programming; fibrosis; lactation; left ventricular hypertrophy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Fetal Undernutrition Modifies Vascular RAS Balance Enhancing Oxidative Damage and Contributing to Remodeling.Int J Mol Sci. 2022 Jan 22;23(3):1233. doi: 10.3390/ijms23031233. Int J Mol Sci. 2022. PMID: 35163158 Free PMC article.
-
Long term effects of fetal undernutrition on rat heart. Role of hypertension and oxidative stress.PLoS One. 2017 Feb 17;12(2):e0171544. doi: 10.1371/journal.pone.0171544. eCollection 2017. PLoS One. 2017. PMID: 28212445 Free PMC article.
-
Role of fetal nutrient restriction and postnatal catch-up growth on structural and mechanical alterations of rat aorta.J Physiol. 2018 Dec;596(23):5791-5806. doi: 10.1113/JP275030. Epub 2018 Jan 31. J Physiol. 2018. PMID: 29277911 Free PMC article.
-
Role of intracardiac renin-angiotensin-aldosterone system in extracellular matrix remodeling.Methods Find Exp Clin Pharmacol. 2003 Sep;25(7):541-64. doi: 10.1358/mf.2003.25.7.778094. Methods Find Exp Clin Pharmacol. 2003. PMID: 14571285 Review.
-
The renin-angiotensin system and experimental heart failure.Cardiovasc Res. 1999 Sep;43(4):838-49. doi: 10.1016/s0008-6363(99)00145-5. Cardiovasc Res. 1999. PMID: 10615411 Review.
Cited by
-
Fetal programming and lactation: modulating gene expression in response to undernutrition during intrauterine life.Pediatr Res. 2024 Jun;95(7):1764-1774. doi: 10.1038/s41390-024-03042-5. Epub 2024 Feb 7. Pediatr Res. 2024. PMID: 38326476
-
Fetal Undernutrition Modifies Vascular RAS Balance Enhancing Oxidative Damage and Contributing to Remodeling.Int J Mol Sci. 2022 Jan 22;23(3):1233. doi: 10.3390/ijms23031233. Int J Mol Sci. 2022. PMID: 35163158 Free PMC article.
-
Renin-Angiotensin System in Liver Metabolism: Gender Differences and Role of Incretins.Metabolites. 2022 May 3;12(5):411. doi: 10.3390/metabo12050411. Metabolites. 2022. PMID: 35629915 Free PMC article. Review.
References
-
- Gray C., Li M., Patel R., Reynolds C., Vickers M. Let-7 miRNA Profiles Are Associated With the Reversal of Left Ventricular Hypertrophy and Hypertension in Adult Male Offspring From Mothers Undernourished During Pregnancy After Preweaning Growth Hormone Treatment. Endocrinology. 2014;155:4808–4817. doi: 10.1210/en.2014-1567. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
