Is an Immunosuppressive Microenvironment a Characteristic of Both Intra- and Extraparenchymal Central Nervous Tumors?
- PMID: 35366268
- PMCID: PMC8830452
- DOI: 10.3390/pathophysiology28010004
Is an Immunosuppressive Microenvironment a Characteristic of Both Intra- and Extraparenchymal Central Nervous Tumors?
Abstract
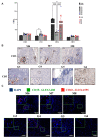
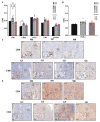
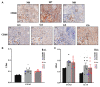
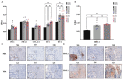
In spite of intensive research, the survival rates of patients diagnosed with tumors of the central nervous system (CNS) have not improved significantly in the last decade. Immunotherapy as novel and efficacious treatment option in several other malignancies has failed in neuro-oncology likely due to the immunosuppressive property of the brain tissues. Glioblastoma (GBM) is the most aggressive malignant CNS neoplasm, while meningioma (MNG) is a mainly low grade or benign brain tumor originating from the non-glial tissues of the CNS. The aim of the current preliminary study is to compare the immune microenvironment of MNG and GBM as potential target in immunotherapy. Interestingly, the immune microenvironment of MNG and GBM have proved to be similar. In both tumors types the immune suppressive elements including regulatory T cells (Treg), tumor-associated macrophages (TAM) were highly elevated. The cytokine environment supporting Treg differentiation and the presence of indoleamine 2,3-dioxygenase 1 (IDO1) have also increased the immunosuppressive microenvironment. The results of the present study show an immune suppressive microenvironment in both brain tumor types. In a follow-up study with a larger patient cohort can provide detailed background information on the immune status of individual patients and aid selection of the best immune checkpoint inhibitor or other immune modulatory therapy. Immune modulatory treatments in combination with IDO1 inhibitors might even become alternative therapy for relapsed, multiple and/or malignant MNG or chemo-resistant GBM.
Keywords: glioblastoma; immune microenvironment; immune suppression; meningioma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
