Interaction of TLK1 and AKTIP as a Potential Regulator of AKT Activation in Castration-Resistant Prostate Cancer Progression
- PMID: 35366279
- PMCID: PMC8830441
- DOI: 10.3390/pathophysiology28030023
Interaction of TLK1 and AKTIP as a Potential Regulator of AKT Activation in Castration-Resistant Prostate Cancer Progression
Abstract
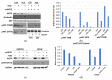
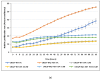
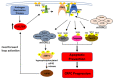
Prostate cancer (PCa) progression is characterized by the emergence of resistance to androgen deprivation therapy (ADT). AKT/PKB has been directly implicated in PCa progression, often due to the loss of PTEN and activation of PI3K>PDK1>AKT signaling. However, the regulatory network of AKT remains incompletely defined. Here, we describe the functional significance of AKTIP in PCa cell growth. AKTIP, identified in an interactome analysis as a substrate of TLK1B (that itself is elevated following ADT), enhances the association of AKT with PDK1 and its phosphorylation at T308 and S473. The interaction between TLK1 and AKTIP led to AKTIP phosphorylation at T22 and S237. The inactivation of TLK1 led to reduced AKT phosphorylation, which was potentiated with AKTIP knockdown. The TLK1 inhibitor J54 inhibited the growth of the LNCaP cells attributed to reduced AKT activation. However, LNCaP cells that expressed constitutively active, membrane-enriched Myr-AKT (which is expected to be active, even in the absence of AKTIP) were also growth-inhibited with J54. This suggested that other pathways (like TLK1>NEK1>YAP) regulating proliferation are also suppressed and can mediate growth inhibition, despite compensation by Myr-AKT. Nonetheless, further investigation of the potential role of TLK1>AKTIP>AKT in suppressing apoptosis, and conversely its reversal with J54, is warranted.
Keywords: AKTIP/FT1/FTS; PKB/AKT; TLK1; apoptosis; castration-resistant prostate cancer; prostate cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Tousled-like kinase 1: a novel factor with multifaceted role in mCRPC progression and development of therapy resistance.Cancer Drug Resist. 2022 Jan 19;5(1):93-101. doi: 10.20517/cdr.2021.109. eCollection 2022. Cancer Drug Resist. 2022. PMID: 35582542 Free PMC article. Review.
-
Generation of Phenothiazine with Potent Anti-TLK1 Activity for Prostate Cancer Therapy.iScience. 2020 Aug 20;23(9):101474. doi: 10.1016/j.isci.2020.101474. eCollection 2020 Sep 25. iScience. 2020. PMID: 32905878 Free PMC article.
-
TLK1>Nek1 Axis Promotes Nuclear Retention and Activation of YAP with Implications for Castration-Resistant Prostate Cancer.Cancers (Basel). 2024 Aug 22;16(16):2918. doi: 10.3390/cancers16162918. Cancers (Basel). 2024. PMID: 39199688 Free PMC article.
-
Beyond the Horizon: Rethinking Prostate Cancer Treatment Through Innovation and Alternative Strategies.Cancers (Basel). 2024 Dec 29;17(1):75. doi: 10.3390/cancers17010075. Cancers (Basel). 2024. PMID: 39796704 Free PMC article.
-
PI3K-AKT-mTOR signaling in prostate cancer progression and androgen deprivation therapy resistance.Asian J Androl. 2014 May-Jun;16(3):378-86. doi: 10.4103/1008-682X.122876. Asian J Androl. 2014. PMID: 24759575 Free PMC article. Review.
Cited by
-
The TLK1-MK5 Axis Regulates Motility, Invasion, and Metastasis of Prostate Cancer Cells.Cancers (Basel). 2022 Nov 22;14(23):5728. doi: 10.3390/cancers14235728. Cancers (Basel). 2022. PMID: 36497211 Free PMC article.
-
Review of Disease-Specific microRNAs by Strategically Bridging Genetics and Epigenetics in Oral Squamous Cell Carcinoma.Genes (Basel). 2023 Aug 2;14(8):1578. doi: 10.3390/genes14081578. Genes (Basel). 2023. PMID: 37628629 Free PMC article. Review.
-
Autophosphorylation of the Tousled-like kinases TLK1 and TLK2 regulates recruitment to damaged chromatin via PCNA interaction.Nucleic Acids Res. 2025 Feb 8;53(4):gkae1279. doi: 10.1093/nar/gkae1279. Nucleic Acids Res. 2025. PMID: 39727191 Free PMC article.
-
Cellular and molecular mechanisms of fibrosis and resolution in bleomycin-induced pulmonary fibrosis mouse model revealed by spatial transcriptome analysis.Heliyon. 2023 Nov 20;9(12):e22461. doi: 10.1016/j.heliyon.2023.e22461. eCollection 2023 Dec. Heliyon. 2023. PMID: 38125541 Free PMC article.
-
Autophosphorylation of the Tousled-like kinases TLK1 and TLK2 regulates recruitment to damaged chromatin via PCNA interaction.bioRxiv [Preprint]. 2024 Apr 26:2024.04.22.590659. doi: 10.1101/2024.04.22.590659. bioRxiv. 2024. Update in: Nucleic Acids Res. 2025 Feb 08;53(4):gkae1279. doi: 10.1093/nar/gkae1279. PMID: 38712247 Free PMC article. Updated. Preprint.
References
-
- Denmeade S.R., Isaacs J.T. Activation of programmed (apoptotic) cell death for the treatment of prostate cancer. Adv. Pharmacol. 1996;35:281–306. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
