Humoral and Cellular Immune Response Elicited by mRNA Vaccination Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in People Living With Human Immunodeficiency Virus Receiving Antiretroviral Therapy Based on Current CD4 T-Lymphocyte Count
- PMID: 35366316
- PMCID: PMC9047161
- DOI: 10.1093/cid/ciac238
Humoral and Cellular Immune Response Elicited by mRNA Vaccination Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in People Living With Human Immunodeficiency Virus Receiving Antiretroviral Therapy Based on Current CD4 T-Lymphocyte Count
Abstract
Background: Data on SARS-CoV-2 vaccine immunogenicity in PLWH are currently limited. Aim of the study was to investigate immunogenicity according to current CD4 T-cell count.
Methods: PLWH on ART attending a SARS-CoV-2 vaccination program, were included in a prospective immunogenicity evaluation after receiving BNT162b2 or mRNA-1273. Participants were stratified by current CD4 T-cell count (poor CD4 recovery, PCDR: <200/mm3; intermediate CD4 recovery, ICDR: 200-500/mm3; high CD4 recovery, HCDR: >500/mm3). RBD-binding IgG, SARS-CoV-2 neutralizing antibodies (nAbs) and IFN-γ release were measured. As control group, HIV-negative healthcare workers (HCWs) were used.
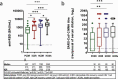
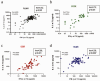
Findings: Among 166 PLWH, after 1 month from the booster dose, detectable RBD-binding IgG were elicited in 86.7% of PCDR, 100% of ICDR, 98.7% of HCDR, and a neutralizing titre ≥1:10 elicited in 70.0%, 88.2%, and 93.1%, respectively. Compared to HCDR, all immune response parameters were significantly lower in PCDR. After adjusting for confounders, current CD4 T-cell <200/mm3 significantly predicted a poor magnitude of anti-RDB, nAbs and IFN-γ response. As compared with HCWs, PCDR elicited a consistently reduced immunogenicity for all parameters, ICDR only a reduced RBD-binding antibody response, whereas HCDR elicited a comparable immune response for all parameters.
Conclusion: Humoral and cell-mediated immune response against SARS-CoV-2 were elicited in most of PLWH, albeit significantly poorer in those with CD4 T-cell <200/mm3 versus those with >500 cell/mm3 and HIV-negative controls. A lower RBD-binding antibody response than HCWs was also observed in PLWH with CD4 T-cell 200-500/mm3, whereas immune response elicited in PLWH with a CD4 T-cell >500/mm3 was comparable to HIV-negative population.
Keywords: 2 vaccine; AIDS; CoV; HIV; SARS; anti; immunogenicity.
© The Author(s) 2022. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures




References
-
- Koff WC, Schenkelberg T, Williams T, et al. Development and deployment of COVID-19 vaccines for those most vulnerable. Sci Transl Med 2021; 13:eabd1525. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

