Tobacco Use and Respiratory Symptoms Among Adults: Findings From the Longitudinal Population Assessment of Tobacco and Health (PATH) Study 2014-2016
- PMID: 35366322
- PMCID: PMC9575972
- DOI: 10.1093/ntr/ntac080
Tobacco Use and Respiratory Symptoms Among Adults: Findings From the Longitudinal Population Assessment of Tobacco and Health (PATH) Study 2014-2016
Erratum in
-
Correction to 20 papers to add an additional interest disclosure.Nicotine Tob Res. 2024 Dec 23;27(1):164. doi: 10.1093/ntr/ntae235. Nicotine Tob Res. 2024. PMID: 39405455 Free PMC article. No abstract available.
Abstract
Introduction: We examined the relationship between current tobacco use and functionally important respiratory symptoms.
Methods: Longitudinal cohort study of 16 295 US adults without COPD in Waves 2-3 (W2-3, 2014-2016) of the Population Assessment of Tobacco and Health Study. Exposure-Ten mutually exclusive categories of tobacco use including single product, multiple product, former, and never use (reference). Outcome-Seven questions assessing wheezing/cough were summed to create a respiratory symptom index; cutoffs of ≥2 and ≥3 were associated with functional limitations and poorer health. Multivariable regressions examined both cutoffs cross-sectionally and change over approximately 12 months, adjusting for confounders.
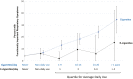
Results: All tobacco use categories featuring cigarettes (>2/3's of users) were associated with higher risk (vs. never users) for functionally important respiratory symptoms at W2, for example, at symptom severity ≥ 3, risk ratio for exclusive cigarette use was 2.34 [95% CI, 1.92, 2.85] and for worsening symptoms at W3 was 2.80 [2.08, 3.76]. There was largely no increased symptom risk for exclusive use of cigars, smokeless tobacco, hookah, or e-cigarettes (adjustment for pack-years and marijuana attenuated the cross-sectional e-cigarette association from 1.53(95% CI 0.98, 2.40) to 1.05 (0.67, 1.63); RRs for these products were also significantly lower compared to exclusive use of cigarettes. The longitudinal e-cigarette-respiratory symptom association was sensitive to the respiratory index cutoff level; exclusive e-cigarette use was associated with worsening symptoms at an index cutoff ≥ 2 (RR = 1.63 [1.02, 2.59]) and with symptom improvement at an index cutoff of ≥ 3 (RR = 1.64 [1.04, 2.58]).
Conclusions: Past and current cigarette smoking drove functionally important respiratory symptoms, while exclusive use of other tobacco products was largely not associated. However, the relationship between e-cigarette use and symptoms was sensitive to adjustment for pack-years and symptom severity.
Implications: How noncigarette tobacco products affect respiratory symptoms is not clear; some studies implicate e-cigarettes. We examined functionally important respiratory symptoms (wheezing/nighttime cough) among US adults without COPD. The majority of adult tobacco users smoke cigarettes and have higher risk of respiratory symptoms and worsening of symptoms, regardless of other products used with them. Exclusive use of other tobacco products (e-cigarettes, cigars, smokeless, hookah) was largely not associated with functionally important respiratory symptoms and risks associated with their use was significantly lower than for cigarettes. The association for e-cigarettes was greatly attenuated by adjustment for cigarette pack-years and sensitive to how symptoms were defined.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Society for Research on Nicotine and Tobacco.
Figures

Comment in
-
E-cigarettes and Respiratory Disorder: The Broader Context.Nicotine Tob Res. 2023 May 22;25(6):1215-1216. doi: 10.1093/ntr/ntad029. Nicotine Tob Res. 2023. PMID: 36812216 Free PMC article. No abstract available.
-
Author Response to E-cigarettes and Respiratory Disorder: The Broader Research Context.Nicotine Tob Res. 2023 May 22;25(6):1217-1218. doi: 10.1093/ntr/ntad036. Nicotine Tob Res. 2023. PMID: 36879402 Free PMC article. No abstract available.
References
-
- US Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
-
- Tashkin DP, Clark VA, Coulson AH, et al. The UCLA population studies of chronic obstructive respiratory disease. VIII. Effects of smoking cessation on lung function: a prospective study of a free-living population. Am Rev Respir Dis. 1984;130(5):707–715. - PubMed
-
- Vasanthi Bathrinarayanan P, Brown JEP, Marshall LJ, Leslie LJ.. An investigation into E-cigarette cytotoxicity in-vitro using a novel 3D differentiated co-culture model of human airways. Toxicol In Vitro. 2018;52:255–264. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

