Improved cognitive impairments by silencing DMP1 via enhancing the proliferation of neural progenitor cell in Alzheimer-like mice
- PMID: 35366382
- PMCID: PMC9124312
- DOI: 10.1111/acel.13601
Improved cognitive impairments by silencing DMP1 via enhancing the proliferation of neural progenitor cell in Alzheimer-like mice
Abstract
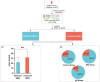
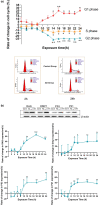
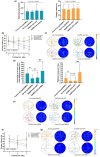
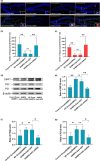
Alzheimer's disease (AD) is age-related progressive neurological dysfunction. Limited clinical benefits for current treatments indicate an urgent need for novel therapeutic strategies. Previous transcriptomic analysis showed that DMP1 expression level was increased in AD model animals whereas it can induce cell-cycle arrest in several cell lines. However, whether the cell-cycle arrest of neural progenitor cell induced by DMP1 affects cognitive function in Alzheimer-like mice still remains unknown. The objective of our study is to explore the issue. We found that DMP1 is correlated with cognitive function based on the clinical genomic analysis of ADNI database. The negative role of DMP1 on neural progenitor cell (NPC) proliferation was revealed by silencing and overexpressing DMP1 in vitro. Furthermore, silencing DMP1 could increase the number of NPCs and improve cognitive function in Alzheimer-like mice, through decreasing P53 and P21 levels, which suggested that DMP1-induced cell-cycle arrest could influence cognitive function.
Keywords: Alzheimer's disease; DMP1; neural progenitor cell; proliferation.
© 2022 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Figures


References
-
- Adamsky, A. , Kol, A. , Kreisel, T. , Doron, A. , Ozeri‐Engelhard, N. , Melcer, T. , Refaeli, R. , Horn, H. , Regev, L. , Groysman, M. , London, M. , & Goshen, I. (2018). Astrocytic activation generates de novo neuronal potentiation and memory enhancement. Cell, 174(1), 59–71.e14. 10.1016/j.cell.2018.05.002 - DOI - PubMed
-
- Bekinschtein, P. , Katche, C. , Slipczuk, L. , Gonzalez, C. , Dorman, G. , Cammarota, M. , Izquierdo, I. , & Medina, J. H. (2010). Persistence of long‐term memory storage: new insights into its molecular signatures in the hippocampus and related structures. Neurotoxicity Research, 18(3–4), 377–385. 10.1007/s12640-010-9155-5 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

