Induction of SARS-CoV-2 neutralizing antibodies by CoronaVac and BNT162b2 vaccines in naïve and previously infected individuals
- PMID: 35366624
- PMCID: PMC8965458
- DOI: 10.1016/j.ebiom.2022.103972
Induction of SARS-CoV-2 neutralizing antibodies by CoronaVac and BNT162b2 vaccines in naïve and previously infected individuals
Abstract
Background: A major challenge of the SARS-CoV-2 pandemic is to better define "protective thresholds" to guide the global response. We aimed to characterize the longitudinal dynamics of the antibody responses in naturally infected individuals in Chile and compared them to humoral responses induced after immunization with CoronaVac-based on an inactivated whole virus -or the BNT162b2- based on mRNA-vaccines. We also contrasted them with the respective effectiveness and efficacy data available for both vaccines.
Methods: We determined and compared the longitudinal neutralizing (nAb) and anti-nucleocapsid (anti-N) antibody responses of 74 COVID-19 individuals (37 outpatient and 37 hospitalized) during the acute disease and convalescence. We also assessed the antibody boosting of 36 of these individuals who were immunized after convalescence with either the CoronaVac (n = 30) or the BNT162b2 (n = 6) vaccines. Antibody titres were also measured for 50 naïve individuals immunized with two doses of CoronaVac (n = 35) or BNT162b2 (n = 15) vaccines. The neutralizing level after vaccination was compared to those of convalescent individuals and the predicted efficacy was estimated.
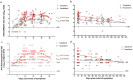
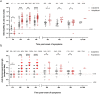
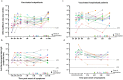
Findings: SARS-CoV-2 infection induced robust nAb and anti-N antibody responses lasting >9 months, but showing a rapid nAb decay. After convalescence, nAb titres were significantly boosted by vaccination with CoronaVac or BNT162b2. In naïve individuals, the calculated mean titre induced by two doses of CoronaVac or BNT162b2 was 0·2 times and 5.2 times, respectively, that of convalescent individuals, which has been proposed as threshold of protection. CoronaVac induced no or only modest anti-N antibody responses. Using two proposed logistic models, the predicted efficacy of BNT162b2 was estimated at 97%, in close agreement with phase 3 efficacy studies, while for CoronaVac it was ∼50% corresponding to the lowest range of clinical trials and below the real-life data from Chile (from February 2 through May 1, 2021 during the predominant circulation of the Gamma variant), where the estimated vaccine effectiveness to prevent COVID-19 was 62·8-64·6%.
Interpretation: The decay of nAbs titres in previously infected individuals over time indicates that vaccination is needed to boost humoral memory responses. Immunization of naïve individuals with two doses of CoronaVac induced nAbs titres that were significantly lower to that of convalescent patients, and similar to vaccination with one dose of BTN162b2. The real life effectiveness for CoronaVac in Chile was higher than estimated; indicating that lower titres and additional cellular immune responses induced by CoronaVac might afford protection in a highly immunized population. Nevertheless, the lower nAb titre induced by two doses of CoronaVac as compared to the BTN162b2 vaccine in naïve individuals, highlights the need of booster immunizations over time to maintain protective levels of antibody, particularly with the emergence of new SARS-CoV-2 variants.
Funding: FONDECYT 1161971, 1212023, 1181799, FONDECYT Postdoctorado 3190706 and 3190648, ANID Becas/Doctorado Nacional 21212258, PIA ACT 1408, CONICYT REDES180170, Centro Ciencia & Vida, FB210008, Financiamiento Basal para Centros Científicos y Tecnológicos de Excelencia grants from the Agencia Nacional de Investigación y Desarrollo (ANID) of Chile; NIH-NIAD grants U19AI135972, R01AI132633 and contracts HHSN272201400008C and 75N93019C00051; the JPB Foundation, the Open Philanthropy Project grant 2020-215611 (5384); and by anonymous donors. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Keywords: COVID-19; Neutralizing antibody persistence; SARS-CoV-2 vaccines; Serological response; Vaccination boost.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The authors reported no potential conflict of interest. The Icahn School of Medicine at Mount Sinai has filed patent applications relating to SARS-CoV-2 serological assays and NDV-based SARS-CoV-2 vaccines which list Florian Krammer as co-inventor. Mount Sinai has spun out a company, Kantaro, to market serological tests for SARS-CoV-2. Florian Krammer has consulted for Merck and Pfizer (before 2020), and is currently consulting for Pfizer, Seqirus, Avimex and Third Rock Ventures. The Krammer laboratory is also collaborating with Pfizer on animal models for SARS-CoV-2. Kartik Chandran is a member of the scientific advisory boards of Integrum Scientific, LLC Biovaxys Technology Corp, and Celdera Medical, LLC; has received royalties from Q2 Solutions and has consulted for Axon Advisors, LLC. Denise Haslwanter, Maria Eugenia Dieterle, Rohit K Jangra and Kartik Chandran, are listed as inventors on a patent application covering the VSV-based SARS2 neutralization assay assigned to Albert Einstein College of Medicine. Rafael Medina has received funding from NIH-Centers of Excellence for Influenza Research and Response (CEIRR) Contract HHSN 75N9301R00028, NIH-Centers of Excellence for Influenza Research and Surveillance (CEIRS) - HHSN272201400008C, the Hope COVID-19 initiative, BHP – UC, FONDECYT 1212023 – ANID Chile and the NIH-NIAID 1U19AI135972: Fluomics: The Next Generation.
Figures

Update of
-
Long-lasting neutralizing antibody responses in SARS-CoV-2 seropositive individuals are robustly boosted by immunization with the CoronaVac and BNT162b2 vaccines.medRxiv [Preprint]. 2021 May 18:2021.05.17.21257197. doi: 10.1101/2021.05.17.21257197. medRxiv. 2021. Update in: EBioMedicine. 2022 Apr;78:103972. doi: 10.1016/j.ebiom.2022.103972. PMID: 34031662 Free PMC article. Updated. Preprint.
References
-
- Post N., Eddy D., Huntley C., et al. Antibody response to SARS-CoV-2 infection in humans: a systematic review. PLoS ONE. 2020;15(12) PubMed PMID: 33382764. PMCID: PMC7775097 www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; JM is chief scientific officer, shareholder and scientific founder of Leucid Bio, a spinout company focused on development of cellular therapeutic agents; no other relationships or activities that could appear to have influenced the submitted work. This does not alter our adherence to PLoS ONE policies on sharing data and materials. Epub 2021/01/01. - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

