Sputum lipoarabinomannan (LAM) as a biomarker to determine sputum mycobacterial load: exploratory and model-based analyses of integrated data from four cohorts
- PMID: 35366820
- PMCID: PMC8976459
- DOI: 10.1186/s12879-022-07308-3
Sputum lipoarabinomannan (LAM) as a biomarker to determine sputum mycobacterial load: exploratory and model-based analyses of integrated data from four cohorts
Abstract
Background: Despite the high global disease burden of tuberculosis (TB), the disease caused by Mycobacterium tuberculosis (Mtb) infection, novel treatments remain an urgent medical need. Development efforts continue to be hampered by the reliance on culture-based methods, which often take weeks to obtain due to the slow growth rate of Mtb. The availability of a "real-time" measure of treatment efficacy could accelerate TB drug development. Sputum lipoarabinomannan (LAM; an Mtb cell wall glycolipid) has promise as a pharmacodynamic biomarker of mycobacterial sputum load.
Methods: The present analysis evaluates LAM as a surrogate for Mtb burden in the sputum samples from 4 cohorts of a total of 776 participants. These include those from 2 cohorts of 558 non-TB and TB participants prior to the initiation of treatment (558 sputum samples), 1 cohort of 178 TB patients under a 14-day bactericidal activity trial with various mono- or multi-TB drug therapies, and 1 cohort of 40 TB patients with data from the first 56-day treatment of a standard 4-drug regimen.
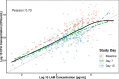
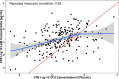
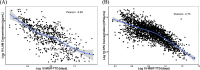
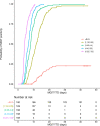
Results: Regression analysis demonstrated that LAM was a predictor of colony-forming unit (CFU)/mL values obtained from the 14-day treatment cohort, with well-estimated model parameters (relative standard error ≤ 22.2%). Moreover, no changes in the relationship between LAM and CFU/mL were observed across the different treatments, suggesting that sputum LAM can be used to reasonably estimate the CFU/mL in the presence of treatment. The integrated analysis showed that sputum LAM also appears to be as good a predictor of time to Mycobacteria Growth Incubator Tube (MGIT) positivity as CFU/mL. As a binary readout, sputum LAM positivity is a strong predictor of solid media or MGIT culture positivity with an area-under-the-curve value of 0.979 and 0.976, respectively, from receiver-operator curve analysis.
Conclusions: Our results indicate that sputum LAM performs as a pharmacodynamic biomarker for rapid measurement of Mtb burden in sputum, and thereby may enable more efficient early phase clinical trial designs (e.g., adaptive designs) to compare candidate anti-TB regimens and streamline dose selection for use in pivotal trials. Trial registration NexGen EBA study (NCT02371681).
Keywords: Biomarker; LAM; Lipoarabinomannan; Mycobacterium; Tuberculosis.
© 2022. The Author(s).
Conflict of interest statement
AB, AGL, AHD, AJ, BK, DA, DH, DHe, JS, KR, LEV, RH, YC, and YL have no competing interests.
GW holds the following 3 patents:
Method for diagnosing tuberculosis: AP/P/2016/009427 (ARIPO), 201580023042.X (China), 15755681.2 (Europe), 201617030869 (India), F/P/2016/258 (Nigeria), 2016/06324 (South Africa).
Serum host biomarkers for tuberculosis disease: PCT/IB2017/052142, due for national filing.
TB diagnostic markers: PCT/IB2013/054377 (USA), host markers in whole blood culture supernatants.
Figures



References
-
- World Health Organization. Global tuberculosis report. 2020.
-
- Bill & Melinda Gates Medical Research Institute. News Release: First-of-Its-Kind Global Collaboration Launched to Develop Transformative Treatment Regimens for Tuberculosis. 2020. https://www.gatesmri.org/news/first-of-its-kind-global-collaboration-lau....
-
- United States Food and Drug Administration. Guidance for industry: pulmonary tuberculosis: developing drugs for treatment. 2015.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

