NADH oxidase of Mycoplasma hyopneumoniae functions as a potential mediator of virulence
- PMID: 35366872
- PMCID: PMC8976378
- DOI: 10.1186/s12917-022-03230-7
NADH oxidase of Mycoplasma hyopneumoniae functions as a potential mediator of virulence
Abstract
Background: Mycoplasma hyopneumoniae (M. hyopneumoniae) is the etiological agent of enzootic pneumonia, a highly infectious swine respiratory disease that distributed worldwide. The pathogenesis and virulence factors of M. hyopneumoniae are not fully clarified. As an important virulence factor of bacteria, nicotinamide adenine dinucleotide (NADH) oxidase (NOX) participates in host-pathogen interaction, however, the function of NOX involved in the pathogenesis of M. hyopneumoniae is not clear.
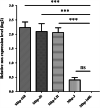
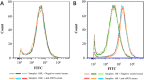
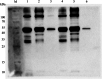
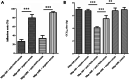
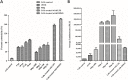
Results: In this study, significant differences in NOX transcription expression levels among different strains of M. hyopneumoniae differed in virulence were identified, suggesting that NOX may be correlated with M. hyopneumoniae virulence. The nox gene of M. hyopneumoniae was cloned and expressed in Escherichia coli, and polyclonal antibodies against recombinant NOX (rNOX) were prepared. We confirmed the enzymatic activity of rNOX based on its capacity to oxidize NADH to NAD+. Flow cytometry analysis demonstrated the surface localization of NOX, and subcellular localization analysis further demonstrated that NOX exists in both the cytoplasm and cell membrane. rNOX was depicted to mediate adhesion to immortalized porcine bronchial epithelial cells (hTERT-PBECs). Pre-neutralizing M. hyopneumoniae with anti-rNOX antibody resulted in a more than 55% reduction in the adhesion rate of high- and low-virulence M. hyopneumoniae strains to hTERT-PBECs. Moreover, a significant difference appeared in the decline in CCU50 titer between virulent (168) and virulence-attenuated (168L) strains. NOX not only recognized and interacted with host fibronectin but also induced cellular oxidative stress and apoptosis in hTERT-PBECs. The release of lactate dehydrogenase by NOX in hTERT-PBECs was positively correlated with the virulence of M. hyopneumoniae strains.
Conclusions: NOX is considered to be a potential virulence factor of M. hyopneumoniae and may play a significant role in mediating its pathogenesis.
Keywords: Adhesion; Mycoplasma hyopneumoniae; NADH oxidase; Virulence factor.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Opriessnig T, Gimenez-Lirola LG, Halbur PG. Polymicrobial respiratory disease in pigs. Anim Health Res Rev. 2011;12(2):133–48. - PubMed
-
- Maes D, Sibila M, Kuhnert P, Segales J, Haesebrouck F, Pieters M. Update on Mycoplasma hyopneumoniae infections in pigs: Knowledge gaps for improved disease control. Transbound Emerg Dis. 2018;65(Suppl 1):110–24. - PubMed
MeSH terms
Substances
Grants and funding
- 2019sy004/the Independent Research Project Program of Jiangsu Key Laboratory for Food Quality and Safety-State Key Laboratory Cultivation Base, Ministry of Science and Technology, China
- CX(21)3124/Jiangsu Agriculture Science and Technology Innovation Fund
- 31800161/National Natural Science Foundation of China
- 31800160/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Research Materials

