Prevalence and factors associated with adverse drug events among patients on dolutegravir-based regimen at the Immune Suppression Syndrome Clinic of Mbarara Regional Referral Hospital, Uganda: a mixed design study
- PMID: 35366917
- PMCID: PMC8976982
- DOI: 10.1186/s12981-022-00442-7
Prevalence and factors associated with adverse drug events among patients on dolutegravir-based regimen at the Immune Suppression Syndrome Clinic of Mbarara Regional Referral Hospital, Uganda: a mixed design study
Abstract
Background: In low income countries such as Uganda progress has been made towards achieving the United Nations AIDS programme 95-95-95 target however efforts are still impeded by pretreatment drug resistance and adverse drug events (ADEs) hence introduction of dolutegravir-based antiretroviral therapy as first-line treatment due to a higher genetic barrier to resistance, better tolerability and safety profile. However, recent studies have raised concerns regarding its safety in real-clinical settings due to ADEs and being a recently introduced drug there is need to actively monitor for ADEs, hence this study aimed to establish the prevalence and factors associated with ADEs among patients on dolutegravir-based regimen at the Immune Suppression Syndrome (ISS) Clinic- Mbarara Regional Referral Hospital (MRRH).
Methods: A mixed design study was conducted at ISS Clinic-MRRH among 375 randomly selected patients who had been exposed to DTG-based regimen for at-least 12 weeks. These were interviewed to obtain data on socio-demographics, dietary habits and their files reviewed for ADEs. Data entry was done using Epi-data 3.0 and exported to SPSS 25.0 for analysis. Prevalence was determined as a percentage, and ADE associated factors assessed using bivariate analysis, those found significant were further subjected to multivariate analysis and considered significant at P < 0.05.
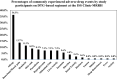
Results: The prevalence of ADEs among patients on DTG-based regimen was found to be 33.1% (124/375) with 5.6% (7/124) participants discontinued from treatment due ADEs, 4 due to hyperglycemia and 3 liver toxicity. The commonly experienced ADE was allergy at 36.3%. Male sex (AOR 1.571, 95% CI 1.433-1.984), WHO stage one at entry to care (AOR 4.586, 95% CI 1.649-12.754), stage two (AOR 4.536, 95% CI 1.611-12.776), stage three (AOR 3.638, 95% CI 1.262-10.488), were significantly associated with ADEs. Patients with undetectable viral load at initiation of DTG-based regimen were 67.6% less likely to experience ADEs (AOR = 0.324, 95% CI 0.1167-0.629).
Conclusion: This study reports a prevalence of 33.1% of ADEs among patients on DTG-based regimen. The most commonly experienced ADE was allergy. Male sex, early HIV disease stage at entry into care and detectable viral load at initiation of DTG-based regimen were significantly associated with ADEs. It is crucial to actively monitor patients with these characteristics for ADEs.
Keywords: Adverse drug events; Dolutegravir-based; HIV; Uganda.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Prevalence of overweight and obesity among adolescents living with HIV after dolutegravir - based antiretroviral therapy start in Kampala, Uganda.AIDS Res Ther. 2024 Apr 18;21(1):23. doi: 10.1186/s12981-024-00615-6. AIDS Res Ther. 2024. PMID: 38637785 Free PMC article.
-
Prevalence of neuropsychiatric adverse events and associated factors among adult patients on dolutegravir attending Mulago ISS clinic.HIV Med. 2023 Apr;24(4):491-501. doi: 10.1111/hiv.13428. Epub 2022 Nov 6. HIV Med. 2023. PMID: 36336827
-
Provider perspectives on the acceptability and tolerability of dolutegravir-based anti-retroviral therapy after national roll-out in Uganda: a qualitative study.BMC Infect Dis. 2021 Dec 7;21(1):1222. doi: 10.1186/s12879-021-06933-8. BMC Infect Dis. 2021. PMID: 34876050 Free PMC article.
-
Doing More With Less: Review of Dolutegravir-Lamivudine, a Novel Single-Tablet Regimen for Antiretroviral-Naïve Adults With HIV-1 Infection.Ann Pharmacother. 2020 Dec;54(12):1252-1259. doi: 10.1177/1060028020933772. Epub 2020 Jun 9. Ann Pharmacother. 2020. PMID: 32517480 Review.
-
Perspectives on the Barrier to Resistance for Dolutegravir + Lamivudine, a Two-Drug Antiretroviral Therapy for HIV-1 Infection.AIDS Res Hum Retroviruses. 2020 Jan;36(1):13-18. doi: 10.1089/AID.2019.0171. Epub 2019 Oct 21. AIDS Res Hum Retroviruses. 2020. PMID: 31507204 Free PMC article. Review.
Cited by
-
Prevalence and Patterns of Adverse Drug Events Among Adult Patients with Human Immune Virus Infection on Dolutegravir-Based Antiretroviral Drug Regimens in Amhara Comprehensive Specialized Hospitals, Northwest Ethiopia: A Multicenter Retrospective Follow-Up Study.HIV AIDS (Auckl). 2023 Jun 1;15:271-278. doi: 10.2147/HIV.S411948. eCollection 2023. HIV AIDS (Auckl). 2023. PMID: 37283816 Free PMC article.
-
Patients reported neuropsychiatric adverse events and associated factors among PLHIV patients receiving DTG-based regimen antiretroviral therapy real-life clinical practice in Ethiopia: multi center crossetional study.BMC Psychiatry. 2025 Apr 16;25(1):383. doi: 10.1186/s12888-025-06820-5. BMC Psychiatry. 2025. PMID: 40241041 Free PMC article.
-
Variation of adverse drug events in different settings in Africa: a systematic review.Eur J Med Res. 2024 Jun 16;29(1):333. doi: 10.1186/s40001-024-01934-0. Eur J Med Res. 2024. PMID: 38880895 Free PMC article.
-
Low Aspartate Aminotransferase/Alanine Aminotransferase Ratio as an Indicator of Metabolic Syndrome Among HIV Patients on Dolutegravir Therapy in Southwestern Uganda.Cureus. 2025 Jan 8;17(1):e77166. doi: 10.7759/cureus.77166. eCollection 2025 Jan. Cureus. 2025. PMID: 39925575 Free PMC article.
-
Nevirapine-Induced Stevens-Johnson Syndrome in an HIV-Infected Patient: A Case Report From Uganda.Int Med Case Rep J. 2025 Feb 13;18:249-253. doi: 10.2147/IMCRJ.S508884. eCollection 2025. Int Med Case Rep J. 2025. PMID: 39963116 Free PMC article.
References
-
- UNAIDS. Understanding fast-track: accelerating action to end the AIDS epidemic by 2030. https://www.unaids.org/sites/default/files/media_asset/201506_JC2743_Und.... Accessed 18 Jan 2022.
-
- UNAIDS. Global HIV & AIDS statistics—2020 fact sheet; 2020. https://www.unaids.org/en/resources/fact-sheet. Accessed 23 Dec 2021.
-
- UNAIDS. UNAIDS DATA 2020; 2020. https://www.unaids.org/en/resources/documents/2020/unaids-data-2020. Accessed 23 Dec Oct 2021.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical