Prevalence and factors associated with adverse drug events among patients on dolutegravir-based regimen at the Immune Suppression Syndrome Clinic of Mbarara Regional Referral Hospital, Uganda: a mixed design study
- PMID: 35366917
- PMCID: PMC8976982
- DOI: 10.1186/s12981-022-00442-7
Prevalence and factors associated with adverse drug events among patients on dolutegravir-based regimen at the Immune Suppression Syndrome Clinic of Mbarara Regional Referral Hospital, Uganda: a mixed design study
Abstract
Background: In low income countries such as Uganda progress has been made towards achieving the United Nations AIDS programme 95-95-95 target however efforts are still impeded by pretreatment drug resistance and adverse drug events (ADEs) hence introduction of dolutegravir-based antiretroviral therapy as first-line treatment due to a higher genetic barrier to resistance, better tolerability and safety profile. However, recent studies have raised concerns regarding its safety in real-clinical settings due to ADEs and being a recently introduced drug there is need to actively monitor for ADEs, hence this study aimed to establish the prevalence and factors associated with ADEs among patients on dolutegravir-based regimen at the Immune Suppression Syndrome (ISS) Clinic- Mbarara Regional Referral Hospital (MRRH).
Methods: A mixed design study was conducted at ISS Clinic-MRRH among 375 randomly selected patients who had been exposed to DTG-based regimen for at-least 12 weeks. These were interviewed to obtain data on socio-demographics, dietary habits and their files reviewed for ADEs. Data entry was done using Epi-data 3.0 and exported to SPSS 25.0 for analysis. Prevalence was determined as a percentage, and ADE associated factors assessed using bivariate analysis, those found significant were further subjected to multivariate analysis and considered significant at P < 0.05.
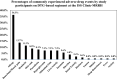
Results: The prevalence of ADEs among patients on DTG-based regimen was found to be 33.1% (124/375) with 5.6% (7/124) participants discontinued from treatment due ADEs, 4 due to hyperglycemia and 3 liver toxicity. The commonly experienced ADE was allergy at 36.3%. Male sex (AOR 1.571, 95% CI 1.433-1.984), WHO stage one at entry to care (AOR 4.586, 95% CI 1.649-12.754), stage two (AOR 4.536, 95% CI 1.611-12.776), stage three (AOR 3.638, 95% CI 1.262-10.488), were significantly associated with ADEs. Patients with undetectable viral load at initiation of DTG-based regimen were 67.6% less likely to experience ADEs (AOR = 0.324, 95% CI 0.1167-0.629).
Conclusion: This study reports a prevalence of 33.1% of ADEs among patients on DTG-based regimen. The most commonly experienced ADE was allergy. Male sex, early HIV disease stage at entry into care and detectable viral load at initiation of DTG-based regimen were significantly associated with ADEs. It is crucial to actively monitor patients with these characteristics for ADEs.
Keywords: Adverse drug events; Dolutegravir-based; HIV; Uganda.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- UNAIDS. Understanding fast-track: accelerating action to end the AIDS epidemic by 2030. https://www.unaids.org/sites/default/files/media_asset/201506_JC2743_Und.... Accessed 18 Jan 2022.
-
- UNAIDS. Global HIV & AIDS statistics—2020 fact sheet; 2020. https://www.unaids.org/en/resources/fact-sheet. Accessed 23 Dec 2021.
-
- UNAIDS. UNAIDS DATA 2020; 2020. https://www.unaids.org/en/resources/documents/2020/unaids-data-2020. Accessed 23 Dec Oct 2021.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical