Odronextamab, a human CD20×CD3 bispecific antibody in patients with CD20-positive B-cell malignancies (ELM-1): results from the relapsed or refractory non-Hodgkin lymphoma cohort in a single-arm, multicentre, phase 1 trial
- PMID: 35366963
- PMCID: PMC10681157
- DOI: 10.1016/S2352-3026(22)00072-2
Odronextamab, a human CD20×CD3 bispecific antibody in patients with CD20-positive B-cell malignancies (ELM-1): results from the relapsed or refractory non-Hodgkin lymphoma cohort in a single-arm, multicentre, phase 1 trial
Abstract
Background: Odronextamab is a hinge-stabilised, fully human IgG4-based CD20 × CD3 bispecific antibody that binds CD3 on T cells and CD20 on B cells. We aimed to evaluate the safety and antitumour activity of odronextamab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma.
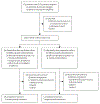
Methods: This single-arm, multicentre, phase 1, dose-escalation and dose-expansion (ELM-1) trial was conducted at ten academic sites across the USA and Germany. Patients aged 18 years or older with CD20-positive relapsed or refractory B-cell malignancies who previously received CD20-directed antibody therapy and who had at least one measurable lesion, and an ECOG performance status of 0 or 1 were included. Patients received intravenous odronextamab, according to a step-up dosing schedule in cycle 1, followed by treatment once per week at target doses ranging from 0·1 mg to 320 mg during cycles 2-4 (each cycle was 21 days). After cycle 4, maintenance treatment occurred every 2 weeks until disease progression or unacceptable toxicity. The primary endpoint of safety was assessed by the incidence of adverse events and dose-limiting toxicities to determine the maximum tolerated dose or phase 2 dose of odronextamab, or both. Preliminary antitumour activity, as measured by objective response rate, was a secondary endpoint. This study is registered with ClinicalTrials.gov, NCT02290951.
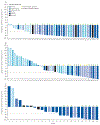
Findings: From Feb 4, 2015, to Sept 25, 2021, 145 heavily pretreated patients (median of 3 (IQR 2-5] previous therapies) were enrolled (94 to the dose-escalation and 51 to the dose-expansion part of the study). The median age of patients was 67·0 years (IQR 57·0-73·0); 101 (70%) were male and 44 (30%) were female; most participants were White (119 [82%]) and not Hispanic or Latino (132 [91%]). 42 (29%) patients received previous CAR T therapy and 119 (82%) were refractory to the last line of therapy. Median duration of follow-up was 4·2 months (IQR 1·5-11·5). During dose escalation, odronextamab was administered up to the maximum dose of 320 mg once per week and no dose-limiting toxicities were observed. The recommended dose for expansion in patients with follicular lymphoma grade 1-3a was 80 mg and was 160 mg for patients with diffuse large B-cell lymphoma. Cytokine release syndrome and neurological treatment-emergent adverse events were predominantly low grade and did not result in treatment discontinuation. The most common grade 3 or worse treatment-emergent adverse events were anaemia (36 [25%]), lymphopenia (28 [19%]), hypophosphataemia (27 [19%]), neutropenia (27 [19%]), and thrombocytopenia (20 [14%]). Serious treatment-emergent adverse events occurred in 89 (61%) of 145 patients; the most frequent were cytokine release syndrome (41 [28%]), pyrexia (11 [8%]), pneumonia (nine [6%]), and infusion-related reaction (six [4%]). Four deaths were considered related to treatment (gastric perforation in a patient with gastric involvement by lymphoma, lung infection, pneumonia, and tumour-lysis syndrome). Objective response rate was 51% (95% CI 42-59; 72 of 142). In patients with follicular lymphoma who received odronextamab doses of 5 mg or higher, the objective response rate was 91% (95% CI 75-98; 29 of 32) and the complete response rate was 72% (95% CI 53-86; 23 of 32). In patients with diffuse large B-cell lymphoma without previous CAR T-cell therapy who received doses of 80 mg or higher, the objective response rate was 53% (eight of 15) and all responses were complete responses. In patients with diffuse large B-cell lymphoma who had previous CAR T-cell therapy and received doses of 80 mg or higher, the objective response rate was 33% (ten of 30) and complete response rate was 27% (eight of 30).
Interpretation: Odronextamab monotherapy showed a manageable safety profile and encouraging preliminary activity, including durable responses in heavily pretreated patients with B-cell non-Hodgkin lymphoma, supporting further clinical investigation in phase 2 and 3 trials.
Funding: Regeneron Pharmaceuticals.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests RB reports research funding from Regeneron Pharmaceuticals, paid to his institution; institutional payments for sponsored studies from AbbVie, Genentech, Pharmacyclics, and Roche; non-financial participation on advisory boards for Janssen Biotech; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from the 18th Annual International Ultmann Chicago Lymphoma Symposium and Curio Science–opinions in diffuse large B cell lymphoma; and his spouse holds stock options and is an employee of Sanofi-Pasteur. JEA reports participation on a data safety monitoring board or advisory board for Bristol Myers Squibb, Juno, and Regeneron Pharmaceuticals. RHA reports advisory board membership from ADC Therapeutics, Bristol Myers Squibb, Epizyme, Genentech, Gilead, Incyte, Karyopharm, Portola, and Seattle Genetics; and institutional research funding from Cyteir, Forty Seven, Genentech, Gilead, Janssen, Regeneron Pharmaceuticals, and Roche. JRB reports sponsored research funding paid to her institution from Gilead, Lilly (Loxo), SecuraBio (Verastem), Sun, and TG Therapeutics; consulting fees from AbbVie, Acerta, AstraZeneca, BeiGene, Bristol Myers Squibb, Catapult Therapeutics, Celgene, Dynamo Therapeutics, Eli Lilly and Company, Gilead, Genentech, Hutchmed, Janssen, Juno, Kite, Loxo, MEI Pharma, Morphosys, Nextcea, Novartis, Octapharma, Pfizer, Pharmacyclics, Rigel, Roche, Sunesis, TG Therapeutics, and Verastem; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Janssen; and participation on a data safety monitoring board or advisory board for Invectys and Morphosys. JNA reports grants or contracts from Celgene, Genentech, Janssen, and TG Therapeutics, paid to his institution; consulting fees from AbbVie, ADC Therapeutics, Ascentage, AstraZeneca, BeiGene, Epizyme, Genentech, Janssen, Pharmacyclics, and TG Therapeutics; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AbbVie, AstraZeneca, BeiGene, Janssen, and PCYC; payment for expert testimony from Calderhead and Lockemeyer Peschke; support for attending meetings and travel from AstraZeneca, BeiGene, and Janssen; and non-financial participation on a data safety monitoring board or advisory board for AstraZeneca. SMO reports consulting fees from AbbVie, Alexion, Amgen, Aptose Biosciences, Astellas, Autolus, Bristol Myers Squibb, Celgene, Eli Lilly and Company, GlaxoSmithKline, Janssen Oncology, Johnson and Johnson, Juno Therapeutics, Merck, NOVA Research, Vaniam, Verastem, and Vida Ventures; research support from Acerta, Caribou, Kite, and Regeneron Pharmaceuticals; and consultant and research support from Gilead, Pfizer, Pharmacyclics, Sunesis, and TG Therapeutics. JCC reports consulting fees from AbbVie, ADC Therapeutics, Janssen, Kymera, Novartis, and TenBio; and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AstraZeneca, BeiGene, Bristol Myers Squibb, Epizyme, and Morphosys. JLC reports research funding from AbbVie, Bayer, Merck, and Roche; and participation on a data safety monitoring board or advisory board for Incyte and Karyopharm. IL reports patents from Regeneron Pharmaceuticals for tumour treatment methods using CD20xCD3 bispecific antibody (US10662244) and a dosing strategy that mitigates cytokine release syndrome for therapeutic antibodies (US20200129617); and holds stock or stock options and is an employee of Regeneron Pharmaceuticals. MST reports research funding from Regeneron Pharmaceuticals; consulting fees from Gilead, Janssen, Kite, Novartis, Regeneron Pharmaceuticals, and Roche; and support for attending meetings or travel from Amgen and Janssen, or both. MU, JL, MZ, SRA, and AC hold stock or stock options for and are employees of Regeneron Pharmaceuticals. All other authors declare no competing interests.
Figures
Comment in
-
Challenges in the development of bispecific antibodies for non-Hodgkin lymphoma.Lancet Haematol. 2022 May;9(5):e314-e315. doi: 10.1016/S2352-3026(22)00104-1. Epub 2022 Apr 1. Lancet Haematol. 2022. PMID: 35366964 No abstract available.
References
-
- Hutchings M, Mous R, Clausen MR, et al. Dose escalation of subcutaneous epcoritamab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an open-label, phase 1/2 study. Lancet 2021; 398: 1157–69. - PubMed
-
- Schuster SJ. Bispecific antibodies for the treatment of lymphomas: promises and challenges. Hematol Oncol 2021; 39 (suppl 1): 113–16. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

