Proteomic elucidation of the targets and primary functions of the picornavirus 2A protease
- PMID: 35367208
- PMCID: PMC9168619
- DOI: 10.1016/j.jbc.2022.101882
Proteomic elucidation of the targets and primary functions of the picornavirus 2A protease
Abstract
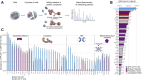
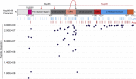
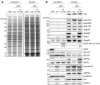
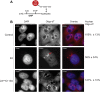
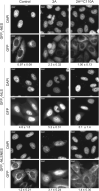
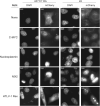
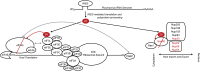
Picornaviruses are small RNA viruses that hijack host cell machinery to promote their replication. During infection, these viruses express two proteases, 2Apro and 3Cpro, which process viral proteins. They also subvert a number of host functions, including innate immune responses, host protein synthesis, and intracellular transport, by utilizing poorly understood mechanisms for rapidly and specifically targeting critical host proteins. Here, we used proteomic tools to characterize 2Apro interacting partners, functions, and targeting mechanisms. Our data indicate that, initially, 2Apro primarily targets just two cellular proteins: eukaryotic translation initiation factor eIF4G (a critical component of the protein synthesis machinery) and Nup98 (an essential component of the nuclear pore complex, responsible for nucleocytoplasmic transport). The protease appears to employ two different cleavage mechanisms; it likely interacts with eIF3L, utilizing the eIF3 complex to proteolytically access the eIF4G protein but also directly binds and degrades Nup98. This Nup98 cleavage results in only a marginal effect on nuclear import of proteins, while nuclear export of proteins and mRNAs were more strongly affected. Collectively, our data indicate that 2Apro selectively inhibits protein translation, key nuclear export pathways, and cellular mRNA localization early in infection to benefit viral replication at the expense of particular cell functions.
Keywords: mass spectrometry; nuclear pore; nuclear transport; picornavirus; plus-stranded RNA virus; protease; proteomics.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures

References
-
- Murphy P.M. Viral exploitation and subversion of the immune system through chemokine mimicry. Nat. Immunol. 2001;2:116–122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

