Mitochondria-associated myosin 19 processively transports mitochondria on actin tracks in living cells
- PMID: 35367209
- PMCID: PMC9065997
- DOI: 10.1016/j.jbc.2022.101883
Mitochondria-associated myosin 19 processively transports mitochondria on actin tracks in living cells
Abstract
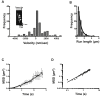
Mitochondria are fundamentally important in cell function, and their malfunction can cause the development of cancer, cardiovascular disease, and neuronal disorders. Myosin 19 (Myo19) shows discrete localization with mitochondria and is thought to play an important role in mitochondrial dynamics and function; however, the function of Myo19 in mitochondrial dynamics at the cellular and molecular levels is poorly understood. Critical missing information is whether Myo19 is a processive motor that is suitable for transportation of mitochondria. Here, we show for the first time that single Myo19 molecules processively move on actin filaments and can transport mitochondria in cells. We demonstrate that Myo19 dimers having a leucine zipper processively moved on cellular actin tracks in demembraned cells with a velocity of 50 to 60 nm/s and a run length of ∼0.4 μm, similar to the movement of isolated mitochondria from Myo19 dimer-transfected cells on actin tracks, suggesting that the Myo19 dimer can transport mitochondria. Furthermore, we show single molecules of Myo19 dimers processively moved on single actin filaments with a large step size of ∼34 nm. Importantly, WT Myo19 single molecules without the leucine zipper processively move in filopodia in living cells similar to Myo19 dimers, whereas deletion of the tail domain abolished such active movement. These results suggest that Myo19 can processively move on actin filaments when two Myo19 monomers form a dimer, presumably as a result of tail-tail association. In conclusion, Myo19 molecules can directly transport mitochondria on actin tracks within living cells.
Keywords: TIRF microscopy; intracellular movement; mitochondria; single molecule; unconventional myosin.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures







References
-
- Coluccio L.M. Springer; Dordrecht: 2008. Myosins: A Superfamily of Molecular Motors.
-
- Yu C., Feng W., Wei Z., Miyanoiri Y., Wen W., Zhao Y., Zhang M. Myosin VI undergoes cargo-mediated dimerization. Cell. 2009;138:537–548. - PubMed
-
- Umeki N., Jung H.S., Sakai T., Sato O., Ikebe R., Ikebe M. Phospholipid-dependent regulation of the motor activity of myosin X. Nat. Struct. Mol. Biol. 2011;18:783–788. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

