Single Ascending Dose Study of a Short Interfering RNA Targeting Lipoprotein(a) Production in Individuals With Elevated Plasma Lipoprotein(a) Levels
- PMID: 35368052
- PMCID: PMC8978050
- DOI: 10.1001/jama.2022.5050
Single Ascending Dose Study of a Short Interfering RNA Targeting Lipoprotein(a) Production in Individuals With Elevated Plasma Lipoprotein(a) Levels
Abstract
Importance: Lipoprotein(a) (Lp[a]) is an important risk factor for atherothrombotic cardiovascular disease and aortic stenosis, for which there are no treatments approved by regulatory authorities.
Objectives: To assess adverse events and tolerability of a short interfering RNA (siRNA) designed to reduce hepatic production of apolipoprotein(a) and to assess associated changes in plasma concentrations of Lp(a) at different doses.
Design, setting, and participants: A single ascending dose study of SLN360, an siRNA targeting apolipoprotein(a) synthesis conducted at 5 clinical research unit sites located in the US, United Kingdom, and Australia. The study enrolled adults with Lp(a) plasma concentrations of 150 nmol/L or greater at screening and no known clinically overt cardiovascular disease. Participants were enrolled between November 18, 2020, and July 21, 2021, with last follow-up on December 29, 2021.
Interventions: Participants were randomized to receive placebo (n = 8) or single doses of SLN360 at 30 mg (n = 6), 100 mg (n = 6), 300 mg (n = 6), or 600 mg (n = 6), administered subcutaneously.
Main outcomes and measures: The primary outcome was evaluation of safety and tolerability. Secondary outcomes included change in plasma concentrations of Lp(a) to a maximum follow-up of 150 days.
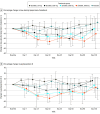
Results: Among 32 participants who were randomized and received the study intervention (mean age, 50 [SD, 13.5] years; 17 women [53%]), 32 (100%) completed the trial. One participant experienced 2 serious adverse event episodes: admission to the hospital for headache following SARS-CoV-2 vaccination and later for complications of cholecystitis, both of which were judged to be unrelated to study drug. Median baseline Lp(a) concentrations were as follows: placebo, 238 (IQR, 203-308) nmol/L; 30-mg SLN360, 171 (IQR, 142-219) nmol/L; 100-mg SLN360, 217 (IQR, 202-274) nmol/L; 300-mg SLN360, 285 (IQR, 195-338) nmol/L; and 600-mg SLN360, 231 (IQR, 179-276) nmol/L. Maximal median changes in Lp(a) were -20 (IQR, -61 to 3) nmol/L, -89 (IQR, -119 to -61) nmol/L, -185 (IQR, -226 to -163) nmol/L, -268 (IQR, -292 to -189) nmol/L, and -227 (IQR, -270 to -174) nmol/L, with maximal median percentage changes of -10% (IQR, -16% to 1%), -46% (IQR, -64% to -40%), -86% (IQR, -92% to -82%), -96% (IQR, -98% to -89%), and -98% (IQR, -98% to -97%), for the placebo group and the 30-mg, 100-mg, 300-mg, and 600-mg SLN360 groups, respectively. The duration of Lp(a) lowering was dose dependent, persisting for at least 150 days after administration.
Conclusions and relevance: In this phase 1 study of 32 participants with elevated Lp(a) levels and no known cardiovascular disease, the siRNA SLN360 was well tolerated, and a dose-dependent lowering of plasma Lp(a) concentrations was observed. The findings support further study to determine the safety and efficacy of this siRNA.
Trial registration: ClinicalTrials.gov Identifier: NCT04606602; EudraCT Identifier: 2020-002471-35.
Conflict of interest statement
Figures

Comment in
-
The Potential Clinical Benefit of Lowering Lipoprotein(a).JAMA. 2022 May 3;327(17):1653-1655. doi: 10.1001/jama.2022.5333. JAMA. 2022. PMID: 35368050 No abstract available.
-
Novel lipid-lowering therapies targeting ANGPTL3 and Lp(a).Nat Rev Cardiol. 2022 Jun;19(6):349. doi: 10.1038/s41569-022-00715-8. Nat Rev Cardiol. 2022. PMID: 35449408 No abstract available.
References
-
- Burgess S, Ference BA, Staley JR, et al. ; European Prospective Investigation Into Cancer and Nutrition–Cardiovascular Disease Consortium . Association of LPA variants with risk of coronary disease and the implications for lipoprotein(a)-lowering therapies: a mendelian randomization analysis. JAMA Cardiol. 2018;3(7):619-627. doi: 10.1001/jamacardio.2018.1470 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

