Protrudin regulates FAK activation, endothelial cell migration and angiogenesis
- PMID: 35368213
- PMCID: PMC8977271
- DOI: 10.1007/s00018-022-04251-z
Protrudin regulates FAK activation, endothelial cell migration and angiogenesis
Abstract
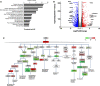
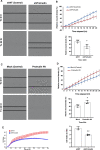
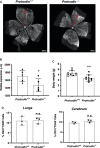
During angiogenesis, endothelial cells form protrusive sprouts and migrate towards the angiogenic stimulus. In this study, we investigate the role of the endoplasmic reticulum (ER)-anchored protein, Protrudin, in endothelial cell protrusion, migration and angiogenesis. Our results demonstrate that Protrudin regulates angiogenic tube formation in primary endothelial cells, Human umbilical vein endothelial cells (HUVECs). Analysis of RNA sequencing data and its experimental validation revealed cell migration as a prominent cellular function affected in HUVECs subjected to Protrudin knockdown. Further, our results demonstrate that knockdown of Protrudin inhibits focal adhesion kinase (FAK) activation in HUVECs and human aortic endothelial cells (HAECs). This is associated with a loss of polarized phospho-FAK distribution upon Protrudin knockdown as compared to Protrudin expressing HUVECs. Reduction of Protrudin also results in a perinuclear accumulation of mTOR and a decrease in VEGF-mediated S6K activation. However, further experiments suggest that the observed inhibition of angiogenesis in Protrudin knockdown cells is not affected by mTOR disturbance. Therefore, our findings suggest that defects in FAK activation and its abnormal subcellular distribution upon Protrudin knockdown are associated with a detrimental effect on endothelial cell migration and angiogenesis. Furthermore, mice with global Protrudin deletion demonstrate reduced retinal vascular progression. To conclude, our results provide evidence for a novel key role of Protrudin in endothelial cell migration and angiogenesis.
Keywords: Endosomes; Focal adhesion kinase; Vasculature; Zfyve27; mTOR.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest concerning this study.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

