A Maximum-Use Trial of Ruxolitinib Cream in Adolescents and Adults with Atopic Dermatitis
- PMID: 35368221
- PMCID: PMC9142470
- DOI: 10.1007/s40257-022-00690-3
A Maximum-Use Trial of Ruxolitinib Cream in Adolescents and Adults with Atopic Dermatitis
Abstract
Background: Ruxolitinib cream is a topical formulation of ruxolitinib, an inhibitor of Janus kinase 1 and Janus kinase 2.
Objective: We aimed to determine the safety, tolerability, and bioavailability of 1.5% ruxolitinib cream under maximum-use conditions in patients with atopic dermatitis. Efficacy was evaluated as an exploratory objective.
Methods: Eligible patients aged ≥ 12-65 years with atopic dermatitis, an Investigator's Global Assessment score ≥ 2, and ≥ 25% affected body surface area were enrolled in an open-label, maximum-use phase I study conducted in the USA and Canada. Patients applied 1.5% ruxolitinib cream twice daily to lesions identified at baseline for the first 28 days and continued use only on active lesions for an additional 28 days (extension period). Safety was assessed by frequency, duration, and severity of treatment-emergent adverse events. Plasma concentrations of ruxolitinib and pharmacokinetic parameters were assessed as secondary endpoints.
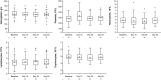
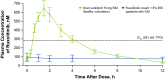
Results: Overall, 41 patients (median age, 17 years; 51% male) were enrolled and 37 (90.2%) entered the extension period, all of whom completed the study. Treatment-emergent adverse events were reported in 13 patients (31.7%). Treatment-related adverse events were reported in four patients (9.8%). The mean (standard deviation) steady-state plasma concentration was 104 (309) nM during the first 28 days, well below the half-maximal inhibitory concentration of Janus kinase-mediated myelosuppression in the bone marrow (281 nM), and decreased further during the extension period. Higher plasma concentrations were detected in a few patients who were treated for a very high affected body surface area. At day 56, 94.6% of patients achieved ≥ 75% improvement in the Eczema Area and Severity Index.
Conclusions: Under maximum-use conditions, ruxolitinib cream was generally well tolerated, with approximately one-third of patients experiencing treatment-emergent adverse events and few treatment-related adverse events. The mean steady-state plasma concentration of ruxolitinib was well below the level expected to affect bone marrow production of blood cells, with a small number of patients exhibiting higher plasma concentrations. In addition, ruxolitinib cream showed a high level of efficacy in patients with atopic dermatitis involving ≥ 25% affected body surface area.
Gov identifier: NCT03920852.
© 2022. The Author(s).
Conflict of interest statement
RB has served as an advisory board member, consultant, speaker, and/or investigator and received honoraria and/or grants from AbbVie, Arcutis, Arena Pharma, Aristea, Asana BioSciences, Bellus Health, Bluefin Biomedicine, Boehringer Ingelheim, CARA, Dermavant, Eli Lilly, EMD Serono, Evidera, Galderma, GlaxoSmithKline, Incyte, Inmagene Bio, Kiniksa, Kyowa Kirin, LEO Pharma, Novan, Pfizer, Ralexar, RAPT Therapeutics, Regeneron, Respivant, Sanofi-Genzyme, Sienna, Target RWE, and Vyne Therapeutics and is an employee and shareholder of Innovaderm Research. RSC has served as a principal investigator for Incyte Corporation. TR has served as an investigator for AbbVie, Arcutis, AstraZeneca, Bausch Health, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Dermira, Galderma, GlaxoSmithKline, Eli Lilly, Incyte Corporation, Janssen, LEO Pharma, Pfizer, Regeneron, Sanofi-Genzyme, and UCB Biopharma. ZZ was an employee and shareholder of Incyte Corporation at the time of this study. SY and XG are employees and shareholders of Incyte Corporation. ML is an investigator for AbbVie, Arcutis, AstraZeneca, Bausch Health, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol Myers Squibb, Dermavant, Dermira, Eli Lilly, Incyte Corporation, Janssen, Kiniksa Pharmaceuticals, LEO Pharma, Pfizer, RAPT Therapeutics, Reistone Biopharma, Regeneron, and UCB Biopharma.
Figures

References
-
- Simpson EL, Bruin-Weller M, Flohr C, Ardern-Jones MR, Barbarot S, Deleuran M, et al. When does atopic dermatitis warrant systemic therapy? Recommendations from an expert panel of the International Eczema Council. J Am Acad Dermatol. 2017;77(4):623–633. doi: 10.1016/j.jaad.2017.06.042. - DOI - PubMed
-
- Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116–132. doi: 10.1016/j.jaad.2014.03.023. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

