Organization of Presynaptic Autophagy-Related Processes
- PMID: 35368245
- PMCID: PMC8968026
- DOI: 10.3389/fnsyn.2022.829354
Organization of Presynaptic Autophagy-Related Processes
Abstract
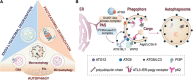
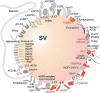
Brain synapses pose special challenges on the quality control of their protein machineries as they are far away from the neuronal soma, display a high potential for plastic adaptation and have a high energy demand to fulfill their physiological tasks. This applies in particular to the presynaptic part where neurotransmitter is released from synaptic vesicles, which in turn have to be recycled and refilled in a complex membrane trafficking cycle. Pathways to remove outdated and damaged proteins include the ubiquitin-proteasome system acting in the cytoplasm as well as membrane-associated endolysosomal and the autophagy systems. Here we focus on the latter systems and review what is known about the spatial organization of autophagy and endolysomal processes within the presynapse. We provide an inventory of which components of these degradative systems were found to be present in presynaptic boutons and where they might be anchored to the presynaptic apparatus. We identify three presynaptic structures reported to interact with known constituents of membrane-based protein-degradation pathways and therefore may serve as docking stations. These are (i) scaffolding proteins of the cytomatrix at the active zone, such as Bassoon or Clarinet, (ii) the endocytic machinery localized mainly at the peri-active zone, and (iii) synaptic vesicles. Finally, we sketch scenarios, how presynaptic autophagic cargos are tagged and recruited and which cellular mechanisms may govern membrane-associated protein turnover in the presynapse.
Keywords: Bassoon; active zone (AZ); amphisome; autophagy; endocytic zone; endolysosomal system; presynaptic proteostasis; synaptic vesicle (SV).
Copyright © 2022 Gundelfinger, Karpova, Pielot, Garner and Kreutz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

