A Model of Combined Exposure to Nicotine and Tetrahydrocannabinol via Electronic Cigarettes in Pregnant Rats
- PMID: 35368251
- PMCID: PMC8966542
- DOI: 10.3389/fnins.2022.866722
A Model of Combined Exposure to Nicotine and Tetrahydrocannabinol via Electronic Cigarettes in Pregnant Rats
Abstract
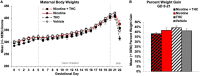
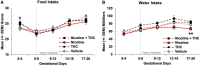
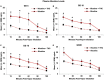
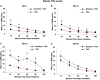
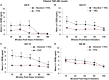
Nicotine and cannabis are two of the most commonly consumed licit and illicit drugs during pregnancy, often consumed together via e-cigarettes. Vaping is assumed to be a safer alternative than traditional routes of consumption, yet the potential consequences of prenatal e-cigarette exposure are largely unknown, particularly when these two drugs are co-consumed. In a novel co-exposure model, pregnant Sprague-Dawley rats received nicotine (36 mg/mL), tetrahydrocannabinol (THC) (100 mg/mL), the combination, or the vehicle via e-cigarettes daily from gestational days 5-20, mimicking the first and second human trimesters. Maternal blood samples were collected throughout pregnancy to measure drug and metabolite levels, and core body temperatures before and after exposure were also measured. Pregnant dams exposed to combined nicotine and THC had lower plasma nicotine and cotinine levels than those exposed to nicotine alone; similarly, the combined exposure group also had lower plasma THC and THC metabolite (THC-OH and THC-COOH) levels than those exposed to THC alone. Prenatal nicotine exposure gradually decreased initial core body temperatures each day, with chronic exposure, whereas exposure to THC decreased temperatures during the individual sessions. Despite these physiological effects, no changes were observed in food or water intake, weight gain, or basic litter outcomes. The use of this model can help elucidate the effects of co-exposure to THC and nicotine via e-cigarettes on both users and their offspring. Understanding the effects of co-use during pregnancy is critical for improving education for pregnant mothers about prenatal e-cigarette use and has important implications for public policy.
Keywords: THC; cannabis; co-exposure; e-cigarette; electronic cigarette; nicotine; poly-drug; prenatal.
Copyright © 2022 Breit, Rodriguez, Hussain, Thomas, Zeigler, Gerasimidis and Thomas.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Abel E. L., Dintcheff B. A. (1978). Effects of prenatal alcohol exposure on growth and development in rats. J. Pharmacol. Exp. Ther. 207 916–921. - PubMed
-
- Andrenyak D. M., Moody D. E., Slawson M. H., O’Leary D. S., Haney M. (2017). Determination of delta-9-tetrahydrocannabinol (THC), 11-hydroxy-THC, 11-nor-9-carboxy-THC and cannabidiol in human plasma using gas chromatography–tandem mass spectrometry. J. Anal. Toxicol. 41 277–288. 10.1093/jat/bkw136 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

