Depression and the Effect of Sertraline on Inflammatory Biomarkers in Patients with Nondialysis CKD
- PMID: 35368605
- PMCID: PMC8809306
- DOI: 10.34067/KID.0000062020
Depression and the Effect of Sertraline on Inflammatory Biomarkers in Patients with Nondialysis CKD
Abstract
Background: Inflammatory biomarkers are elevated in patients with CKD and associated with poor outcomes. Major depressive disorder (MDD) is prevalent in CKD and associated with inflammation. No studies investigated the effect of MDD treatment on plasma inflammatory biomarkers in patients with nondialysis CKD.
Methods: In a prespecified analysis of the randomized, double-blind CKD Antidepressant Sertraline Trial, we investigated whether treatment with sertraline versus placebo or response to treatment would affect plasma levels of albumin, prealbumin, IL-6, and high-sensitivity C-reactive protein (hsCRP), measured at baseline and after 12 weeks of treatment. We also explored whether somatic versus nonsomatic depressive symptoms, measured using the Quick Inventory of Depressive Symptomatology, and quality-of-life subscales, measured using the Kidney Disease Quality of Life Short Form, were associated with baseline levels of these inflammatory biomarkers.
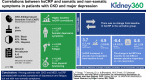
Results: Of the 193 participants, mean age was 58.4 (SD 13) years and 58% were black, 42% were white, and 18% were Hispanic. Higher baseline hsCRP correlated with somatic depressive symptoms (r=0.21; P=0.01), fatigue (r=0.22; P=0.005), and poorer physical functioning (r=-0.26; P=0.001). There was no change in hsCRP in the sertraline group. hsCRP increased in placebo nonresponders from baseline (median, 3.7 mg/L; interquartile range [IQR], 1.7-10.0 mg/L) to exit (median, 4.9 mg/L; IQR, 1.8-8.8 mg/L; P=0.01). The change from baseline to exit differed between placebo responders (median, -0.4 mg/L; IQR, -9.3 to 0.2 mg/L) and nonresponders (median, 0.8 mg/L; IQR, -0.1 to 3.9 mg/L; P=0.008). There were no differences in changes in albumin, prealbumin, or IL-6 from baseline in any group.
Conclusions: Among patients with CKD and MDD, hsCRP correlated with somatic symptoms of depression and fatigue, but not with nonsomatic symptoms. Sertraline treatment was not associated with a longitudinal change in hsCRP from baseline regardless of treatment effect on depressive symptoms, but those who failed to respond to placebo had an increase in hsCRP over time. This area deserves further investigation.
Clinical trial registry name and registration number: CKD Antidepressant Sertraline Trial (CAST), NCT00946998.
Keywords: C-reactive protein; Chronic Kidney Disease; Sertraline; biomarkers; chronic kidney disease; depression; fatigue; high-sensitivity C-reactive protein; inflammation; interleukin-6; major depressive disorder; medically unexplained symptoms; quality of life.
Copyright © 2020 by the American Society of Nephrology.
Conflict of interest statement
M. Trivedi has served as an advisor or consultant to the following organizations: Allergan Sales LLC, Alkermes, Arcadia Pharmaceuticals Inc., AstraZeneca, Axon Advisors, Bristol-Myers Squibb Company, Eli Lilly & Company, Evotec, Johnson & Johnson, Lundbeck, MedAvante, Merck, MSI Methylation Sciences Inc., Nestle Health Science-PamLab Inc., Naurex, Neuronetics, One Carbon Therapeutics Ltd., Otsuka Pharmaceuticals, Roche Products Ltd., SHIRE Development, Takeda, and Tal Medical/Puretech Venture. M. Trivedi reports personal fees from Acadia, Akili Interactive (other), Alto Neuroscience Inc., Applied Clinical Intelligence LLC, Axome Therapeutics, Boehringer Ingelheim, Engage Health Media, GreenLight VitalSign6 Inc., Health Care Global Village, Janssen-Cilag, Janssen Research and Development LLC, Jazz Pharmaceuticals, Medscape LLC, Navitor Pharmaceuticals Inc, Otsuka Pharmaceutical Development & Commercialization Inc., Otsuka America Pharmaceutical Inc, Perception Neuroscience Holdings Inc., Pharmerit International LP, Policy Analysis Inc., Rexahn Pharmaceuticals Inc., Sage Therapeutics, Signant Health, SK Life Science Inc., and The Baldwin Group Inc. He also reports grants from the National Institute of Mental Health, Patient-Centered Outcomes Research Institute, Cancer Prevention Research Institute of Texas, other from Oxford University Press, and other from American Psychiatric Association (Deputy Editor for American Journal of Psychiatry), outside the submitted work. The coauthors have nothing to disclose.
Figures


Similar articles
-
Pharmacologic and psychological interventions for depression treatment in patients with kidney disease.Curr Opin Nephrol Hypertens. 2020 Sep;29(5):457-464. doi: 10.1097/MNH.0000000000000629. Curr Opin Nephrol Hypertens. 2020. PMID: 32701597 Free PMC article. Review.
-
Effect of Sertraline on Depressive Symptoms in Patients With Chronic Kidney Disease Without Dialysis Dependence: The CAST Randomized Clinical Trial.JAMA. 2017 Nov 21;318(19):1876-1890. doi: 10.1001/jama.2017.17131. JAMA. 2017. PMID: 29101402 Free PMC article. Clinical Trial.
-
Association of platelet function with depression and its treatment with sertraline in patients with chronic kidney disease: analysis of a randomized trial.BMC Nephrol. 2019 Oct 29;20(1):395. doi: 10.1186/s12882-019-1576-7. BMC Nephrol. 2019. PMID: 31664940 Free PMC article. Clinical Trial.
-
Association of obesity and inflammatory marker levels on treatment outcome: results from a double-blind, randomized study of adjunctive L-methylfolate calcium in patients with MDD who are inadequate responders to SSRIs.J Clin Psychiatry. 2015 Dec;76(12):1635-41. doi: 10.4088/JCP.14m09587. J Clin Psychiatry. 2015. PMID: 26613389 Clinical Trial.
-
A systematic review and meta-analysis of the evidence on inflammation in depressive illness and symptoms in chronic and end-stage kidney disease.Psychol Med. 2023 Sep;53(12):5839-5851. doi: 10.1017/S0033291722003099. Epub 2022 Oct 18. Psychol Med. 2023. PMID: 36254747
Cited by
-
Navigating the intersection of mental health and kidney health: a systematic review of antidepressant safety in renal impairment.Discov Ment Health. 2025 Mar 17;5(1):36. doi: 10.1007/s44192-025-00163-z. Discov Ment Health. 2025. PMID: 40097730 Free PMC article. Review.
-
Inflammatory Determinants and Associated Morbidity in Hemodialysis Patients.J Pers Med. 2023 Aug 27;13(9):1311. doi: 10.3390/jpm13091311. J Pers Med. 2023. PMID: 37763079 Free PMC article.
-
Unveiling the Anti-Inflammatory Effects of Antidepressants: A Systematic Review of Human Studies over the Last Decade.Pharmaceuticals (Basel). 2025 Jun 10;18(6):867. doi: 10.3390/ph18060867. Pharmaceuticals (Basel). 2025. PMID: 40573262 Free PMC article. Review.
-
Pharmacologic and psychological interventions for depression treatment in patients with kidney disease.Curr Opin Nephrol Hypertens. 2020 Sep;29(5):457-464. doi: 10.1097/MNH.0000000000000629. Curr Opin Nephrol Hypertens. 2020. PMID: 32701597 Free PMC article. Review.
-
Fatigue in CKD: Epidemiology, Pathophysiology, and Treatment.Clin J Am Soc Nephrol. 2021 Sep;16(9):1445-1455. doi: 10.2215/CJN.19891220. Epub 2021 Apr 15. Clin J Am Soc Nephrol. 2021. PMID: 33858827 Free PMC article. Review.
References
-
- Hedayati SS, Bosworth HB, Briley LP, Sloane RJ, Pieper CF, Kimmel PL, Szczech LA: Death or hospitalization of patients on chronic hemodialysis is associated with a physician-based diagnosis of depression. Kidney Int 74: 930–936, 2008 - PubMed
-
- Kimmel PL, Peterson RA, Weihs KL, Simmens SJ, Alleyne S, Cruz I, Veis JH: Multiple measurements of depression predict mortality in a longitudinal study of chronic hemodialysis outpatients. Kidney Int 57: 2093–2098, 2000 - PubMed
-
- Palmer SC, Vecchio M, Craig JC, Tonelli M, Johnson DW, Nicolucci A, Pellegrini F, Saglimbene V, Logroscino G, Hedayati SS, Strippoli GF: Association between depression and death in people with CKD: A meta-analysis of cohort studies. Am J Kidney Dis 62: 493–505, 2013 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

