Improvement of Mesenchymal Stromal Cell Proliferation and Differentiation via Decellularized Extracellular Matrix on Substrates With a Range of Surface Chemistries
- PMID: 35368802
- PMCID: PMC8969767
- DOI: 10.3389/fmedt.2022.834123
Improvement of Mesenchymal Stromal Cell Proliferation and Differentiation via Decellularized Extracellular Matrix on Substrates With a Range of Surface Chemistries
Abstract
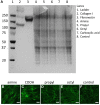
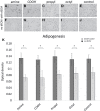
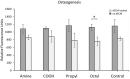
Decellularized extracellular matrix (dECM) deposited by mesenchymal stromal cells (MSCs) has emerged as a promising substrate for improved expansion of MSCs. To date, essentially all studies that have produced dECM for MSC expansion have done so on tissue culture plastic or glass. However, substrate surface chemistry has a profound impact on the adsorption of proteins that mediate cell-material interactions, and different surface chemistries can cause changes in cell behavior, ECM deposition, and the in vivo response to a material. This study tested the hypothesis that substrate surface chemistry impacts the deposition of ECM and its subsequent bioactivity. This hypothesis was tested by producing glass surfaces with various surface chemistries (amine, carboxylic acid, propyl, and octyl groups) using silane chemistry. ECM was deposited by an immortalized MSC line, decellularized, and characterized through SDS-PAGE and immunofluorescence microscopy. No significant difference was observed in dECM composition or microarchitecture on the different surfaces. The decellularized surfaces were seeded with primary MSCs and their proliferation and differentiation were assessed. The presence of dECM improved the proliferation of primary MSCs by ~100% in comparison to surface chemistry controls. Additionally, the adipogenesis increased by 50-90% on all dECM surfaces in comparison to surface chemistry controls, and the osteogenesis increased by ~50% on the octyl-modified surfaces when dECM was present. However, no statistically significant differences were observed within the set of dECM surfaces or control surfaces. These results support the null hypothesis, meaning surface chemistry (over the range tested in this work) is not a key regulator of the composition or bioactivity of MSC-derived dECM. These results are significant because they provide an important insight into regenerative engineering technologies. Specifically, the utilization of dECM in stem cell manufacturing and tissue engineering applications would require the dECM to be produced on a wide variety of substrates. This work indicates that it can be produced on materials with a range of surface chemistries without undesired changes in the bioactivity of the dECM.
Keywords: biomaterial; cell secreted matrix; decellularized extracellular matrix; mesenchymal stem cell; mesenchymal stromal cell; stem cell manufacturing; surface chemistry; tissue engineering.
Copyright © 2022 Yang, O'Connor, Kalionis and Heath.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Tailored environments for directed mesenchymal stromal cell proliferation and differentiation using decellularized extracellular matrices in conjunction with substrate modulus.Acta Biomater. 2024 Oct 1;187:110-122. doi: 10.1016/j.actbio.2024.08.022. Epub 2024 Aug 22. Acta Biomater. 2024. PMID: 39181177
-
Native and solubilized decellularized extracellular matrix: A critical assessment of their potential for improving the expansion of mesenchymal stem cells.Acta Biomater. 2017 Jun;55:1-12. doi: 10.1016/j.actbio.2017.04.014. Epub 2017 Apr 12. Acta Biomater. 2017. PMID: 28412553 Review.
-
Mesenchymal Stromal Cell-Produced Components of Extracellular Matrix Potentiate Multipotent Stem Cell Response to Differentiation Stimuli.Front Cell Dev Biol. 2020 Sep 22;8:555378. doi: 10.3389/fcell.2020.555378. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33072743 Free PMC article.
-
Decellularized extracellular matrices produced from immortal cell lines derived from different parts of the placenta support primary mesenchymal stem cell expansion.PLoS One. 2017 Feb 2;12(2):e0171488. doi: 10.1371/journal.pone.0171488. eCollection 2017. PLoS One. 2017. PMID: 28152107 Free PMC article.
-
Age associated communication between cells and matrix: a potential impact on stem cell-based tissue regeneration strategies.Organogenesis. 2014;10(3):289-98. doi: 10.4161/15476278.2014.970089. Organogenesis. 2014. PMID: 25482504 Free PMC article. Review.
Cited by
-
Therapeutic potential of extracellular vesicles for treating human pregnancy disorders.Extracell Vesicles Circ Nucl Acids. 2025 Jun 12;6(2):287-309. doi: 10.20517/evcna.2025.07. eCollection 2025. Extracell Vesicles Circ Nucl Acids. 2025. PMID: 40852594 Free PMC article. Review.
-
MSCs-Derived Decellularised Matrix: Cellular Responses and Regenerative Dentistry.Int Dent J. 2024 Jun;74(3):403-417. doi: 10.1016/j.identj.2024.02.011. Epub 2024 Mar 16. Int Dent J. 2024. PMID: 38494389 Free PMC article. Review.
-
Atomic force microscopy for characterization of decellularized extracellular matrix (dECM) based materials.Sci Technol Adv Mater. 2024 Oct 29;25(1):2421739. doi: 10.1080/14686996.2024.2421739. eCollection 2024. Sci Technol Adv Mater. 2024. PMID: 39559530 Free PMC article. Review.
References
-
- DiGirolamo C, Stokes D, Colter D, Phinney D, Class R, Prockop D. Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol. (1999) 107:275–81. 10.1046/j.1365-2141.1999.01715.x - DOI - PubMed
LinkOut - more resources
Full Text Sources

