Hypomagnesemia in the Cancer Patient
- PMID: 35368816
- PMCID: PMC8785729
- DOI: 10.34067/KID.0005622020
Hypomagnesemia in the Cancer Patient
Abstract
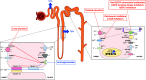
Hypomagnesemia is a common medical problem that contributes to the morbidity and mortality of patients with cancer. This review summarizes magnesium physiology and highlights the mechanisms underlying magnesium disturbances due to cancer and cancer treatment. The causes of hypomagnesemia can be categorized according to the pathophysiologic mechanism: decreased intake, transcellular shift, gastrointestinal losses, and kidney losses. Patients with cancer are at risk for opportunistic infections, frequently experience cardiovascular complications, and often receive classes of medications that cause or exacerbate hypomagnesemia. Also, cancer-specific therapies are responsible for hypomagnesemia, including platinum-based chemotherapy, anti-EGF receptor mAbs, human EGF receptor-2 target inhibitors (HER2), and calcineurin inhibitors. Urinary indices, such as the fractional excretion of magnesium, can provide useful information about the etiology. The management of hypomagnesemia depends on the magnitude of hypomagnesemia and the underlying cause. We recommended checking serum magnesium at the beginning of treatment and as part of routine monitoring throughout cancer treatment. Opportunities exist for potential research and practice improvement, including further characterization of hypomagnesemia regarding the clinical effect on cancer outcomes, preventing hypomagnesemia in patients receiving high-risk anticancer agents, and developing effective therapeutic strategies.
Keywords: TRPM6; acid/base and electrolyte disorders; cetuximab; cisplatin; electrolytes; hypomagnesemia; magnesium; neoplasms; onconephrology.
Copyright © 2021 by the American Society of Nephrology.
Conflict of interest statement
K.D. Jhaveri is a consultant for Astex Pharmaceuticals and Natera; is a paid contributor to Uptodate.com; and reports receiving honorarium from the International Society of Nephrology and the American Society of Nephrology. B.T. Workeneh reports being on a speakers bureau for AstraZeneca. All remaining authors have nothing to disclose.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

