The Microbiome and p-Inulin in Hemodialysis: A Feasibility Study
- PMID: 35369018
- PMCID: PMC8786005
- DOI: 10.34067/KID.0006132020
The Microbiome and p-Inulin in Hemodialysis: A Feasibility Study
Abstract
Background: The intestinal microbiome is an appealing target for interventions in ESKD because of its likely contribution to uremic toxicity. Before conducting clinical trials of microbiome-altering treatments, it is necessary to understand the within-person and between-person variability in the composition and function of the gut microbiome in patients with ESKD.
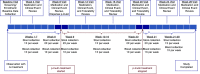
Methods: We conducted a multicenter, nonrandomized, crossover feasibility study of patients on maintenance hemodialysis consisting of three phases: pretreatment (8 weeks); treatment, during which the prebiotic, p-inulin, was administered at a dosage of 8 g twice daily (12 weeks); and post-treatment (8 weeks). Stool samples were collected 1-2 times per week and blood was collected weekly for 28 weeks. The gut microbiome was characterized using 16S ribosomal-RNA sequencing and metabolomic profiling.
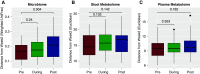
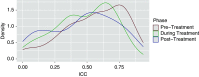
Results: A total of 11 of the 13 participants completed the 28-week study. Interparticipant variability was greater than intraparticipant variability for microbiome composition (P<0.001 by UniFrac distances) and metabolomic composition (P<0.001 by Euclidean distances). p-Inulin was well tolerated by 12 of 13 participants. Adherence to the frequent sample collection and self-aliquoting of stool samples were both 96%. A change in the microbiome composition from pretreatment to post-treatment was evident by the overall shifts in weighted UniFrac distances (P=0.004) and a progressive decrease in prevalence of high intraclass correlations, indicating an increase in intraparticipant microbiome diversity during and after p-inulin treatment. An effect of p-inulin on the metabolomic profile was not evident.
Conclusions: The intraparticipant stability of the gut microbiome under no-treatment conditions, the tolerability of p-inulin, the signals of increased diversity of the microbiome with p-inulin treatment, and the willingness of participants to provide stool samples all support the feasibility of a larger trial to investigate interventions targeting the gut microbiome in patients with ESKD. Whether or not p-inulin has sufficient efficacy as an intervention requires evaluation in larger studies.
Clinical trial registry name and registration number: Gut Microbiome and p-Inulin in Hemodialysis, NCT02572882.
Keywords: crossover trial; dialysis; feasibility studies; hemodialysis; microbiome; p-inulin; prebiotic.
Copyright © 2021 by the American Society of Nephrology.
Conflict of interest statement
D.M. Charytan reports receiving personal fees from AstraZeneca, Douglas and London, Fresenius, GSK, Merck, PLC Medical, and Zoll; grants and personal fees from Amgen, Gilead, Medtronic, and NovoNordisk; grants from Bioporto; other from Daichi-Sankyo; and personal fees and other from Janssen, outside the submitted work. L.M. Dember receives consulting fees from GlaxoSmithKline and Merck, and compensation from the National Kidney Foundation for her role as deputy editor of American Journal of Kidney Diseases, outside of the submitted work. J. Himmelfarb reports being a founder of AKTIV-X Technologies, Inc., with equity; and has received fees for acting as a consultant or scientific advisory board member for Akebia, Chinook Therapeutics, Maze Therapeutics, Pfizer, Renalytix AI, and Seattle Genetics. T.A. Ikizler received personal fees from Abbott Renal Care and Fresenius Kabi, during the conduct of the study. P.L. Kimmel is a coeditor of Chronic Renal Disease (Academic Press, San Diego, CA), and a member of the board of directors of the Washington Academy of Medicine. A.S. Kliger receives income from the American Society of Nephrology, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and Yale New Haven Hospital. H. Li receives consulting fees from Eli Lily, outside the submitted work. R. Mehrotra receives consulting fees from Baxter Healthcare, outside the submitted work. All remaining authors have nothing to disclose.
Figures