A Standardized Extract of Lentinula edodes Cultured Mycelium Inhibits Pseudomonas aeruginosa Infectivity Mechanisms
- PMID: 35369436
- PMCID: PMC8966770
- DOI: 10.3389/fmicb.2022.814448
A Standardized Extract of Lentinula edodes Cultured Mycelium Inhibits Pseudomonas aeruginosa Infectivity Mechanisms
Erratum in
-
Corrigendum: A standardized extract of Lentinula edodes cultured mycelium inhibits Pseudomonas aeruginosa infectivity mechanisms.Front Microbiol. 2023 Mar 28;14:1183760. doi: 10.3389/fmicb.2023.1183760. eCollection 2023. Front Microbiol. 2023. PMID: 37056749 Free PMC article.
Abstract
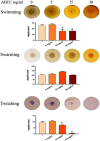
The priority pathogen list of the World Health Organization classified Pseudomonas aeruginosa as the second top critical pathogen. Hence, the development of novel antibacterial strategies to tackle this bacterium is highly necessary. Herein we explore the potential antibacterial effect of a standardized extract of cultured mycelium of Lentinula edodes (AHCC®) on P. aeruginosa. AHCC® was found to inhibit the growth rate and biofilm formation of strain PAO1. No change in swarming was observed, but AHCC® hampered swimming and twitching motility. In accordance, a decreased expression of metabolism, growth, and biofilm formation genes was shown. AHCC® also diminished the levels of exotoxin A and bacteria inside IEC18 cells and the secretion of IL-6, IL-10 and TNF by infected macrophages. This effect was related to a reduced phosphorylation of MAPKs and to bacteria internalization. Taken together, our data suggest that AHCC® has a potential role to prevent P. aeruginosa infections and may lead to the development of new therapies.
Keywords: AHCC®; PCR real time (qPCR); Pseudomonas aeruginosa; immune response; internalization; motility and biofilm; prebiotic; secretion system and adhesion.
Copyright © 2022 Tena-Garitaonaindia, Ceacero-Heras, Montoro, de Medina, Martínez-Augustin and Daddaoua.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Abril M. A., Michan C., Timmis K. N., Ramos J. L. (1989). Regulator and enzyme specificities of the TOL plasmid-encoded upper pathway for degradation of aromatic hydrocarbons and expansion of the substrate range of the pathway. J. Bacteriol. 171 6782–6790. 10.1128/jb.171.12.6782-6790.1989 - DOI - PMC - PubMed

