Development of a Rapid and Efficient RPA-CRISPR/Cas12a Assay for Mycoplasma pneumoniae Detection
- PMID: 35369478
- PMCID: PMC8965353
- DOI: 10.3389/fmicb.2022.858806
Development of a Rapid and Efficient RPA-CRISPR/Cas12a Assay for Mycoplasma pneumoniae Detection
Abstract
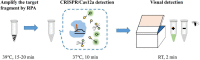
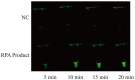
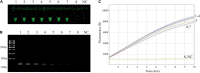
Mycoplasma pneumoniae (MP) is a one of most common pathogen in causing respiratory infection in children and adolescents. Rapid and efficient diagnostic methods are crucial for control and treatment of MP infections. Herein, we present an operationally simple, rapid and efficient molecular method for MP identification, which eliminates expensive instruments and specialized personnel. The method combines recombinase polymerase amplification (RPA) with clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR associated proteins (Cas) 12a-based detection, with an optimal procedure less than 1 h from sample to result including DNA extraction (25 min), RPA reaction (39°C for 15-20 min), CRISPR/Cas12a detection (37°C for 10 min) and visual detection by naked eyes (2 min). This diagnostic method shows high sensitivity (two copies per reaction) and no cross-reactivity against other common pathogenic bacteria. Preliminary evaluation using 201 clinical samples shows sensitivity of 99.1% (107/108), specificity of 100% (93/93) and consistency of 99.5% (200/201), compared with real-time PCR method. The above data demonstrate that our developed method is reliable for rapid diagnosis of MP. In conclusion, the RPA-CRISPR/Cas12a has a great potential to be as a useful tool for reliable and quick diagnosis of MP infection, especially in primary hospitals with limited conditions.
Keywords: CRISPR/Cas12a; Mycoplasma pneumoniae; RPA; recombinase polymerase amplification; visual detection.
Copyright © 2022 Li, Xiao, Yang, Yao, Li, Zheng, Guo, Wang, Chen, Guo, Wang and Shen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

