RELAY+: Exploratory Study of Ramucirumab Plus Gefitinib in Untreated Patients With EGFR-Mutated Metastatic NSCLC
- PMID: 35369607
- PMCID: PMC8966141
- DOI: 10.1016/j.jtocrr.2022.100303
RELAY+: Exploratory Study of Ramucirumab Plus Gefitinib in Untreated Patients With EGFR-Mutated Metastatic NSCLC
Abstract
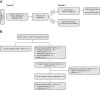
Introduction: Ramucirumab (RAM) plus erlotinib was found to have superior progression-free survival (PFS) versus placebo plus erlotinib in untreated EGFR-mutated metastatic NSCLC in the global phase 3 RELAY study. RELAY+ was an open-label, two-period, single-arm, exploratory study of RAM plus gefitinib (GEF; period 1) and RAM plus osimertinib (period 2) in East Asia (NCT02411448).
Methods: Period 1 evaluated RAM (10 mg/kg) plus GEF (250 mg/d) in patients with untreated EGFR-mutated metastatic NSCLC. Period 2 evaluated RAM plus osimertinib (80 mg/d) in patients with disease progression who acquired T790M mutation in period 1. Exploratory end points included 1-year PFS rate (primary), other efficacy parameters, safety, and biomarker analyses of plasma (baseline, on-treatment, follow-up) using next-generation sequencing.
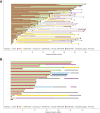
Results: From December 2017 to August 2018, a total of 82 patients were enrolled and started treatment (period 1, RAM + GEF). The 1-year PFS rate was 62.9% (95% confidence interval: 50.3-73.1). Treatment-emergent adverse events of grade three or higher were reported with RAM plus GEF in 60 of 82 patients (73.2%; five patients [6.1%] grade four). There were two deaths owing to adverse events that occurred (acute cardiac failure, congestive cardiac failure). T790M rate at disease progression in plasma was 81.0% (13 of 16 patients).
Conclusions: RELAY+ was found to have a favorable benefit-risk profile for RAM plus GEF in first-line treatment of East Asian patients with EGFR-mutated NSCLC.
Keywords: East Asia; Japan; Plasma biopsy; Treatment outcome; Vascular endothelial growth factor receptor-2.
© 2022 The Authors.
Figures

References
-
- World Health Organization. Globocan 2020 Cancer fact sheets: Eastern Asia. https://gco.iarc.fr/today/data/factsheets/populations/906-eastern-asia-f...
-
- Hsu W.H., Yang J.C., Mok T.S., Loong H.H. Overview of current systemic management of EGFR-mutant NSCLC. Ann Oncol. 2018;29:i3–i9. - PubMed
-
- Shigematsu H., Lin L., Takahashi T., et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

