Effect of Vupanorsen on Non-High-Density Lipoprotein Cholesterol Levels in Statin-Treated Patients With Elevated Cholesterol: TRANSLATE-TIMI 70
- PMID: 35369705
- PMCID: PMC9047643
- DOI: 10.1161/CIRCULATIONAHA.122.059266
Effect of Vupanorsen on Non-High-Density Lipoprotein Cholesterol Levels in Statin-Treated Patients With Elevated Cholesterol: TRANSLATE-TIMI 70
Abstract
Background: Genetic loss-of-function variants in ANGPTL3 are associated with lower levels of plasma lipids. Vupanorsen is a hepatically targeted antisense oligonucleotide that inhibits Angiopoietin-like 3 (ANGPTL3) protein synthesis.
Methods: Adults with non-high-density lipoprotein cholesterol (non-HDL-C) ≥100 mg/dL and triglycerides 150 to 500 mg/dL on statin therapy were randomized in a double-blind fashion to placebo or 1 of 7 vupanorsen dose regimens (80, 120, or 160 mg SC every 4 weeks, or 60, 80, 120, or 160 mg SC every 2 weeks). The primary end point was placebo-adjusted percentage change from baseline in non-HDL-C at 24 weeks. Secondary end points included placebo-adjusted percentage changes from baseline in triglycerides, low-density lipoprotein cholesterol (LDL-C), apolipoprotein B (ApoB), and ANGPTL3.
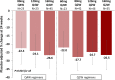
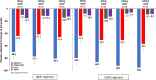
Results: Two hundred eighty-six subjects were randomized: 44 to placebo and 242 to vupanorsen. The median age was 64 (interquartile range, 58-69) years, 44% were female, the median non-HDL-C was 132.4 (interquartile range, 118.0-154.1) mg/dL, and the median triglycerides were 216.2 (interquartile range, 181.4-270.4) mg/dL. Vupanorsen resulted in significant decreases from baseline over placebo in non-HDL-C ranging from 22.0% in the 60 mg every 2 weeks arm to 27.7% in the 80 mg every 2 weeks arm (all P<0.001 for all doses). There were dose-dependent reductions in triglycerides that ranged from 41.3% to 56.8% (all P<0.001). The effects on LDL-C and ApoB were more modest (7.9%-16.0% and 6.0%-15.1%, respectively) and without a clear dose-response relationship' and only the higher reductions achieved statistical significance. ANGPTL3 levels were decreased in a dose-dependent manner by 69.9% to 95.2% (all P<0.001). There were no confirmed instances of significant decline in renal function or platelet count with vupanorsen. Injection site reactions and >3× elevations of alanine aminotransferase or aspartate aminotransferase were more common at higher total monthly doses (up to 33.3% and 44.4%, respectively), and there was a dose-dependent increase in hepatic fat fraction (up to 76%).
Conclusions: Vupanorsen administered at monthly equivalent doses from 80 to 320 mg significantly reduced non-HDL-C and additional lipid parameters. Injection site reactions and liver enzyme elevations were more frequent at higher doses, and there was a dose-dependent increase in hepatic fat fraction.
Registration: URL: https://clinicaltrials.gov; Unique identifier: NCT04516291.
Keywords: Angiopoietin-like proteins; antisense oligonucleotide; lipids; triglycerides.
Figures

Comment in
-
Novel lipid-lowering therapies targeting ANGPTL3 and Lp(a).Nat Rev Cardiol. 2022 Jun;19(6):349. doi: 10.1038/s41569-022-00715-8. Nat Rev Cardiol. 2022. PMID: 35449408 No abstract available.
References
-
- Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, et al. ; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489 - PubMed
-
- Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, et al. ; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664 - PubMed
-
- Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, et al. ; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. doi: 10.1056/NEJMoa1801174 - PubMed
-
- Marston NA, Giugliano RP, Melloni GEM, Park JG, Morrill V, Blazing MA, Ference B, Stein E, Stroes ES, Braunwald E, et al. Association of apolipoprotein B-containing lipoproteins and risk of myocardial infarction in individuals with and without atherosclerosis: distinguishing between particle concentration, type, and content. JAMA Cardiol. 2022;7:250–256. - PMC - PubMed
-
- Johannesen CDL, Mortensen MB, Langsted A, Nordestgaard BG. Apolipoprotein B and non-HDL cholesterol better reflect residual risk than LDL cholesterol in statin-treated patients. J Am Coll Cardiol. 2021;77:1439–1450. doi: 10.1016/j.jacc.2021.01.027 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

