Global diversity of policy, coverage, and demand of COVID-19 vaccines: a descriptive study
- PMID: 35369871
- PMCID: PMC8977121
- DOI: 10.1186/s12916-022-02333-0
Global diversity of policy, coverage, and demand of COVID-19 vaccines: a descriptive study
Abstract
Background: Hundreds of millions of doses of coronavirus disease 2019 (COVID-19) vaccines have been administered globally, but progress on vaccination varies considerably between countries. We aimed to provide an overall picture of COVID-19 vaccination campaigns, including policy, coverage, and demand of COVID-19 vaccines.
Methods: We conducted a descriptive study of vaccination policy and doses administered data obtained from multiple public sources as of 8 February 2022. We used these data to develop coverage indicators and explore associations of vaccine coverage with socioeconomic and healthcare-related factors. We estimated vaccine demand as numbers of doses required to complete vaccination of countries' target populations according to their national immunization program policies.
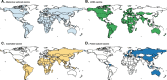
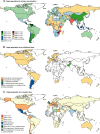
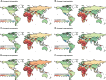
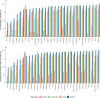
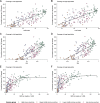
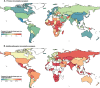
Results: Messenger RNA and adenovirus vectored vaccines were the most commonly used COVID-19 vaccines in high-income countries, while adenovirus vectored vaccines were the most widely used vaccines worldwide (180 countries). One hundred ninety-two countries have authorized vaccines for the general public, with 40.1% (77/192) targeting individuals over 12 years and 32.3% (62/192) targeting those ≥ 5 years. Forty-eight and 151 countries have started additional-dose and booster-dose vaccination programs, respectively. Globally, there have been 162.1 doses administered per 100 individuals in target populations, with marked inter-region and inter-country heterogeneity. Completed vaccination series coverage ranged from 0.1% to more than 95.0% of country target populations, and numbers of doses administered per 100 individuals in target populations ranged from 0.2 to 308.6. Doses administered per 100 individuals in whole populations correlated with healthcare access and quality index (R2 = 0.59), socio-demographic index (R2 = 0.52), and gross domestic product per capita (R2 = 0.61). At least 6.4 billion doses will be required to complete interim vaccination programs-3.3 billion for primary immunization and 3.1 billion for additional/booster programs. Globally, 0.53 and 0.74 doses per individual in target populations are needed for primary immunization and additional/booster dose programs, respectively.
Conclusions: There is wide country-level disparity and inequity in COVID-19 vaccines rollout, suggesting large gaps in immunity, especially in low-income countries.
Keywords: COVID-19 vaccines; Global diversity; Vaccination policy; Vaccine coverage; Vaccine demand.
© 2022. The Author(s).
Conflict of interest statement
H.Y. has received research funding from Sanofi Pasteur, Shanghai Roche Pharmaceutical Company, and SINOVAC Biotech Ltd. Except for research funding from SINOVAC Biotech Ltd, which is related to the data analysis of clinical trials of immunogenicity and safety of CoronaVac, the others are not related to COVID-19. All other authors report no competing interests.
Figures
References
-
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. 2022. https://covid19.who.int/.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

