Microglia: Key Players in Retinal Ageing and Neurodegeneration
- PMID: 35370560
- PMCID: PMC8968040
- DOI: 10.3389/fncel.2022.804782
Microglia: Key Players in Retinal Ageing and Neurodegeneration
Abstract
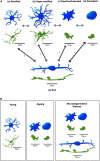
Microglia are the resident immune cells of the central nervous system (CNS) and play a key role in maintaining the normal function of the retina and brain. During early development, microglia migrate into the retina, transform into a highly ramified phenotype, and scan their environment constantly. Microglia can be activated by any homeostatic disturbance that may endanger neurons and threaten tissue integrity. Once activated, the young microglia exhibit a high diversity in their phenotypes as well as their functions, which relate to either beneficial or harmful consequences. Microglial activation is associated with the release of cytokines, chemokines, and growth factors that can determine pathological outcomes. As the professional phagocytes in the retina, microglia are responsible for the clearance of pathogens, dead cells, and protein aggregates. However, their phenotypic diversity and phagocytic capacity is compromised with ageing. This may result in the accumulation of protein aggregates and myelin debris leading to retinal neuroinflammation and neurodegeneration. In this review, we describe microglial phenotypes and functions in the context of the young and ageing retina, and the mechanisms underlying changes in ageing. Additionally, we review microglia-mediated retinal neuroinflammation and discuss the mechanisms of microglial involvement in retinal neurodegenerative diseases.
Keywords: ageing; microglia; morphology; phagocytosis; phenotypes; retina; retinal neurodegenerative disease.
Copyright © 2022 Guo, Choi, Bikkannavar and Cordeiro.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
The Role of Microglia in Retinal Neurodegeneration: Alzheimer's Disease, Parkinson, and Glaucoma.Front Aging Neurosci. 2017 Jul 6;9:214. doi: 10.3389/fnagi.2017.00214. eCollection 2017. Front Aging Neurosci. 2017. PMID: 28729832 Free PMC article. Review.
-
Microglia in Physiology and Disease.Annu Rev Physiol. 2017 Feb 10;79:619-643. doi: 10.1146/annurev-physiol-022516-034406. Epub 2016 Dec 7. Annu Rev Physiol. 2017. PMID: 27959620 Review.
-
Retinal and Brain Microglia in Multiple Sclerosis and Neurodegeneration.Cells. 2021 Jun 15;10(6):1507. doi: 10.3390/cells10061507. Cells. 2021. PMID: 34203793 Free PMC article. Review.
-
Lysosomal acidification dysfunction in microglia: an emerging pathogenic mechanism of neuroinflammation and neurodegeneration.J Neuroinflammation. 2023 Aug 5;20(1):185. doi: 10.1186/s12974-023-02866-y. J Neuroinflammation. 2023. PMID: 37543564 Free PMC article. Review.
-
Translocator protein (18 kDa) (TSPO) is expressed in reactive retinal microglia and modulates microglial inflammation and phagocytosis.J Neuroinflammation. 2014 Jan 8;11:3. doi: 10.1186/1742-2094-11-3. J Neuroinflammation. 2014. PMID: 24397957 Free PMC article.
Cited by
-
Microglia Morphological Response to Mesenchymal Stromal Cell Extracellular Vesicles Demonstrates EV Therapeutic Potential for Modulating Neuroinflammation.bioRxiv [Preprint]. 2024 Jul 3:2024.07.01.601612. doi: 10.1101/2024.07.01.601612. bioRxiv. 2024. Update in: J Biol Eng. 2024 Oct 17;18(1):58. doi: 10.1186/s13036-024-00449-w. PMID: 39005342 Free PMC article. Updated. Preprint.
-
Evaluating therapeutic potential of NR2E3 doses in the rd7 mouse model of retinal degeneration.Sci Rep. 2024 Jul 17;14(1):16490. doi: 10.1038/s41598-024-67095-6. Sci Rep. 2024. PMID: 39019967 Free PMC article.
-
Do multiple physiological OCT biomarkers indicate age-related decline in rod mitochondrial function in C57BL/6J mice?Front Neurosci. 2023 Nov 17;17:1280453. doi: 10.3389/fnins.2023.1280453. eCollection 2023. Front Neurosci. 2023. PMID: 38046657 Free PMC article.
-
Regulation of disease-associated microglia in the optic nerve by lipoxin B4 and ocular hypertension.Mol Neurodegener. 2024 Nov 20;19(1):86. doi: 10.1186/s13024-024-00775-z. Mol Neurodegener. 2024. PMID: 39568070 Free PMC article.
-
Letter to the Editor Regarding "Intraretinal Hyper-Reflective Foci Are Almost Universally Present and Co-Localize With Intraretinal Fluid in Diabetic Macular Edema".Invest Ophthalmol Vis Sci. 2025 Feb 3;66(2):59. doi: 10.1167/iovs.66.2.59. Invest Ophthalmol Vis Sci. 2025. PMID: 39982708 Free PMC article. No abstract available.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

