Aspirin in COVID-19: Pros and Cons
- PMID: 35370686
- PMCID: PMC8965577
- DOI: 10.3389/fphar.2022.849628
Aspirin in COVID-19: Pros and Cons
Abstract
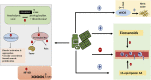
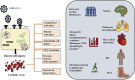
Since its emergence, the COVID-19 pandemic has been ravaging the medical and economic sectors even with the significant vaccination advances. In severe presentations, the disease of SARS-CoV-2 can manifest with life-threatening thromboembolic and multi-organ repercussions provoking notable morbidity and mortality. The pathogenesis of such burdensome forms has been under extensive investigation and is attributed to a state of immune dysfunction and hyperinflammation. In light of these extraordinary circumstances, research efforts have focused on investigating and repurposing previously available agents that target the inflammatory and hematological cascades. Aspirin, due to its well-known properties and multiple molecular targets, and ought to its extensive clinical use, has been perceived as a potential therapeutic agent for COVID-19. Aspirin acts at multiple cellular targets to achieve its anti-inflammatory and anti-platelet effects. Although initial promising clinical data describing aspirin role in COVID-19 has appeared, evidence supporting its use remains fragile and premature. This review explores the notion of repurposing aspirin in COVID-19 infection. It delves into aspirin as a molecule, along with its pharmacology and clinical applications. It also reviews the current high-quality clinical evidence highlighting the role of aspirin in SARS-CoV-2 infection.
Keywords: COVID-19; SARS-CoV-2; aspirin; coronavirus; salicylic acid.
Copyright © 2022 Zareef, Diab, Al Saleh, Makarem, Younis, Bitar and Arabi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Abdelwahab H. W., Shaltout S. W., Sayed Ahmed H. A., Fouad A. M., Merrell E., Riley J. B., et al. (2021). Acetylsalicylic Acid Compared with Enoxaparin for the Prevention of Thrombosis and Mechanical Ventilation in COVID-19 Patients: A Retrospective Cohort Study. Clin. Drug Investig. 41 (8), 723–732. 10.1007/s40261-021-01061-2 - DOI - PMC - PubMed
-
- Aktaa S., Wu J., Nadarajah R., Rashid M., de Belder M., Deanfield J., et al. (2021). Incidence and Mortality Due to Thromboembolic Events during the COVID-19 Pandemic: Multi-Sourced Population-Based Health Records Cohort Study. Thromb. Res. 202, 17–23. 10.1016/j.thromres.2021.03.006 - DOI - PMC - PubMed
-
- Antman E. M., Anbe D. T., Armstrong P. W., Bates E. R., Green L. A., Hand M., et al. (2004). ACC/AHA Guidelines for the Management of Patients with ST-Elevation Myocardial Infarction-Eexecutive Summary: a Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation 110 (3), 588–636. 10.1161/01.CIR.0000134791.68010.FA - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

