Development and Content Validity of the Bilateral Vestibulopathy Questionnaire
- PMID: 35370880
- PMCID: PMC8968143
- DOI: 10.3389/fneur.2022.852048
Development and Content Validity of the Bilateral Vestibulopathy Questionnaire
Abstract
Background: To date, the burden and severity of the full spectrum of bilateral vestibulopathy (BVP) symptoms has not yet been measured in a standardized manner. Since therapeutic interventions aiming to improve BVP symptoms are emerging, the need for a new standardized assessment tool that encompasses the specific aspects of BVP arises. Therefore, the aim of this study was to develop a multi-item Patient Reported Outcome Measure (PROM) that captures the clinically important symptoms of BVP and assesses its impact on daily life.
Methods: The development of the Bilateral Vestibulopathy Questionnaire (BVQ) consisted of two phases: (I) initial item generation and (II) face and content validity testing. Items were derived from a literature review and individual semi-structured interviews focusing on the full spectrum of reported BVP symptoms (I). Subsequently (IIa), individual patient interviews were conducted using "thinking aloud" and concurrent verbal probing techniques to assess the comprehensibility of the instructions, questions and response options, and the relevance, missing domains, or missing items. Interviews continued until saturation of input was reached. Finally, international experts with experience in the field of the physical, emotional, and cognitive symptoms of BVP participated in an online focus group to assess the relevance and comprehensiveness of the BVQ (IIb).
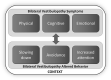
Results: The BVQ consisted of two sections. The first section included 50 items scored on a six-point Likert scale arranged into seven constructs (i.e., imbalance, oscillopsia, other physical symptoms, cognitive symptoms, emotional symptoms, limitations and behavioral changes and social life). The second section consisted of four items, scored on a visual analog scale from 0 to 100, to inquire about limitations in daily life, perceived health and expectations regarding future recovery. Interviews with BVP patients [n = 8, 50% female, mean age 56 years (range 24-88 years)] and the expert meeting confirmed face and content validity of the developed BVQ.
Conclusion: The BVQ, which was developed to assess the spectrum of BVP symptoms and its impact on daily life, proved to have good face and content validity. It can be used to characterize current self-reported symptoms and disability and to evaluate symptom burden before and after therapeutic interventions in future research and clinical practice.
Keywords: Patient Reported Outcome Measure (PROM); bilateral vestibulopathy; questionnaire; symptoms bilateral vestibulopathy; vestibular impairment.
Copyright © 2022 van Stiphout, Hossein, Kimman, Whitney, Ayiotis, Strupp, Guinand, Pérez Fornos, Widdershoven, Ramos-Macías, Van Rompaey and van de Berg.
Conflict of interest statement
LS was supported through funding of MED-EL (Innsbruck, Austria). RB, AP, and NG received funding for travel from MED-EL. MS is Joint Chief Editor of the Journal of Neurology, Editor in Chief of Frontiers of Neuro-otology and Section Editor of F1000 and has received speaker's honoraria from Abbott, Auris Medical, Biogen, Eisai, Grünenthal, GSK, Henning Pharma, Interacoustics, J&J, MSD, NeuroUpdate, Otometrics, Pierre-Fabre, TEVA, UCB, and Viatris. MS also receives support for clinical studies from Decibel, U.S.A., Cure within Reach, U.S.A. and Heel, Germany and acts as a consultant for Abbott, AurisMedical, Heel, IntraBio and Sensorion. SW is a paid consultant for Intelligent Automation (a BlueHalo Company). The funders had no role in study design, data collection, data analysis, interpretation of data, decision to publish, or preparation of the manuscript. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Construct validity and reliability of the Bilateral Vestibulopathy Questionnaire (BVQ).Front Neurol. 2023 Nov 1;14:1221037. doi: 10.3389/fneur.2023.1221037. eCollection 2023. Front Neurol. 2023. PMID: 38020641 Free PMC article.
-
DISCOHAT: An Acronym to Describe the Spectrum of Symptoms Related to Bilateral Vestibulopathy.Front Neurol. 2021 Nov 12;12:771650. doi: 10.3389/fneur.2021.771650. eCollection 2021. Front Neurol. 2021. PMID: 34867759 Free PMC article.
-
Clinical Subtypes and vHIT Parameters in a Population With Bilateral Vestibulopathy.Front Neurol. 2021 Jun 7;12:673974. doi: 10.3389/fneur.2021.673974. eCollection 2021. Front Neurol. 2021. PMID: 34163428 Free PMC article.
-
Can Galvanic Vestibular Stimulation Be an Effective Management for Bilateral Vestibulopathy?Med J Islam Repub Iran. 2022 Mar 7;36:18. doi: 10.47176/mjiri.36.18. eCollection 2022. Med J Islam Repub Iran. 2022. PMID: 35999928 Free PMC article. Review.
-
Noisy galvanic vestibular stimulation: an emerging treatment option for bilateral vestibulopathy.J Neurol. 2017 Oct;264(Suppl 1):81-86. doi: 10.1007/s00415-017-8481-4. Epub 2017 Apr 8. J Neurol. 2017. PMID: 28391373 Review.
Cited by
-
Bilateral vestibulopathy: a clinical update and proposed diagnostic algorithm.Front Neurol. 2023 Dec 19;14:1308485. doi: 10.3389/fneur.2023.1308485. eCollection 2023. Front Neurol. 2023. PMID: 38178884 Free PMC article. Review.
-
Construct validity and reliability of the Bilateral Vestibulopathy Questionnaire (BVQ).Front Neurol. 2023 Nov 1;14:1221037. doi: 10.3389/fneur.2023.1221037. eCollection 2023. Front Neurol. 2023. PMID: 38020641 Free PMC article.
-
The VertiGO! Trial protocol: A prospective, single-center, patient-blinded study to evaluate efficacy and safety of prolonged daily stimulation with a multichannel vestibulocochlear implant prototype in bilateral vestibulopathy patients.PLoS One. 2024 Mar 28;19(3):e0301032. doi: 10.1371/journal.pone.0301032. eCollection 2024. PLoS One. 2024. PMID: 38547135 Free PMC article.
-
Chronic unilateral vestibular hypofunction: a qualitative study exploring the full spectrum of symptoms and impacts through the ICF framework.Front Neurol. 2025 Jun 2;16:1589404. doi: 10.3389/fneur.2025.1589404. eCollection 2025. Front Neurol. 2025. PMID: 40529438 Free PMC article.
-
Cognitive interviewing validation of the Chinese version of the neurogenic bladder symptom score.Heliyon. 2024 Sep 6;10(18):e37435. doi: 10.1016/j.heliyon.2024.e37435. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39309897 Free PMC article.
References
LinkOut - more resources
Full Text Sources

