Do Muscle Changes Contribute to the Neurological Disorder in Spastic Paresis?
- PMID: 35370894
- PMCID: PMC8964436
- DOI: 10.3389/fneur.2022.817229
Do Muscle Changes Contribute to the Neurological Disorder in Spastic Paresis?
Abstract
Background: At the onset of stroke-induced hemiparesis, muscle tissue is normal and motoneurones are not overactive. Muscle contracture and motoneuronal overactivity then develop. Motor command impairments are classically attributed to the neurological lesion, but the role played by muscle changes has not been investigated.
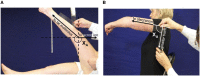
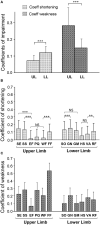
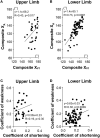
Methods: Interaction between muscle and command disorders was explored using quantified clinical methodology-the Five Step Assessment. Six key muscles of each of the lower and upper limbs in adults with chronic poststroke hemiparesis were examined by a single investigator, measuring the angle of arrest with slow muscle stretch (XV1) and the maximal active range of motion against the resistance of the tested muscle (XA). The coefficient of shortening CSH = (XN-XV1)/XN (XN, normally expected amplitude) and of weakness CW = (XV1-XA)/XV1) were calculated to estimate the muscle and command disorders, respectively. Composite CSH (CCSH) and CW (CCW) were then derived for each limb by averaging the six corresponding coefficients. For the shortened muscles of each limb (mean CSH > 0.10), linear regressions explored the relationships between coefficients of shortening and weakness below and above their median coefficient of shortening.
Results: A total of 80 persons with chronic hemiparesis with complete lower limb assessments [27 women, mean age 47 (SD 17), time since lesion 8.8 (7.2) years], and 32 with upper limb assessments [18 women, age 32 (15), time since lesion 6.4 (9.3) years] were identified. The composite coefficient of shortening was greater in the lower than in the upper limb (0.12 ± 0.04 vs. 0.08 ± 0.04; p = 0.0002, while the composite coefficient of weakness was greater in the upper limb (0.28 ± 0.12 vs. 0.15 ± 0.06, lower limb; p < 0.0001). In the lower limb shortened muscles, the coefficient of weakness correlated with the composite coefficient of shortening above the 0.15 median CSH (R = 0.43, p = 0.004) but not below (R = 0.14, p = 0.40).
Conclusion: In chronic hemiparesis, muscle shortening affects the lower limb particularly, and, beyond a threshold of severity, may alter descending commands. The latter might occur through chronically increased intramuscular tension, and thereby increased muscle afferent firing and activity-dependent synaptic sensitization at the spinal level.
Keywords: chronic hemiparesis; clinical extensibility; muscle disorder; quantified assessment; spastic cocontraction; spastic myopathy; stretch-sensitive paresis; synaptic sensitization.
Copyright © 2022 Pradines, Ghédira, Bignami, Vielotte, Bayle, Marciniak, Burke, Hutin and Gracies.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources

