Aberrant Cerebral Iron Trafficking Co-morbid With Chronic Inflammation: Molecular Mechanisms and Pharmacologic Intervention
- PMID: 35370907
- PMCID: PMC8964494
- DOI: 10.3389/fneur.2022.855751
Aberrant Cerebral Iron Trafficking Co-morbid With Chronic Inflammation: Molecular Mechanisms and Pharmacologic Intervention
Abstract
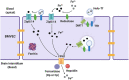
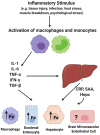
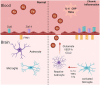
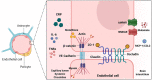
The redox properties that make iron an essential nutrient also make iron an efficient pro-oxidant. Given this nascent cytotoxicity, iron homeostasis relies on a combination of iron transporters, chaperones, and redox buffers to manage the non-physiologic aqueous chemistry of this first-row transition metal. Although a mechanistic understanding of the link between brain iron accumulation (BIA) and neurodegenerative diseases is lacking, BIA is co-morbid with the majority of cognitive and motor function disorders. The most prevalent neurodegenerative disorders, including Alzheimer's Disease (AD), Parkinson's Disease (PD), Multiple System Atrophy (MSA), and Multiple Sclerosis (MS), often present with increased deposition of iron into the brain. In addition, ataxias that are linked to mutations in mitochondrial-localized proteins (Friedreich's Ataxia, Spinocerebellar Ataxias) result in mitochondrial iron accumulation and degradation of proton-coupled ATP production leading to neuronal degeneration. A comorbidity common in the elderly is a chronic systemic inflammation mediated by primary cytokines released by macrophages, and acute phase proteins (APPs) released subsequently from the liver. Abluminal inflammation in the brain is found downstream as a result of activation of astrocytes and microglia. Reasonably, the iron that accumulates in the brain comes from the cerebral vasculature via the microvascular capillary endothelial cells whose tight junctions represent the blood-brain barrier. A premise amenable to experimental interrogation is that inflammatory stress alters both the trans- and para-cellular flux of iron at this barrier resulting in a net accumulation of abluminal iron over time. This review will summarize the evidence that lends support to this premise; indicate the mechanisms that merit delineation; and highlight possible therapeutic interventions based on this model.
Keywords: aging; blood-brain barrier; brain iron; chronic inflammation; iron trafficking; neurodegeneration.
Copyright © 2022 Rosenblum and Kosman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich's ataxia.J Neurol Sci. 2005 Jun 15;233(1-2):145-62. doi: 10.1016/j.jns.2005.03.012. J Neurol Sci. 2005. PMID: 15896810 Review.
-
Cerebral Iron Deposition in Neurodegeneration.Biomolecules. 2022 May 17;12(5):714. doi: 10.3390/biom12050714. Biomolecules. 2022. PMID: 35625641 Free PMC article. Review.
-
Ageing, neuroinflammation and neurodegeneration.Front Biosci (Schol Ed). 2015 Jun 1;7(1):189-204. doi: 10.2741/S433. Front Biosci (Schol Ed). 2015. PMID: 25961695 Review.
-
Lactoferrin for Mental Health: Neuro-Redox Regulation and Neuroprotective Effects across the Blood-Brain Barrier with Special Reference to Neuro-COVID-19.J Diet Suppl. 2023;20(2):218-253. doi: 10.1080/19390211.2021.1922567. Epub 2021 May 12. J Diet Suppl. 2023. PMID: 33977807
-
Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells.J Neurochem. 2013 Aug;126(4):541-9. doi: 10.1111/jnc.12244. Epub 2013 Apr 3. J Neurochem. 2013. PMID: 23506423
Cited by
-
Iron Overload in Brain: Transport Mismatches, Microbleeding Events, and How Nanochelating Therapies May Counteract Their Effects.Int J Mol Sci. 2024 Feb 16;25(4):2337. doi: 10.3390/ijms25042337. Int J Mol Sci. 2024. PMID: 38397013 Free PMC article. Review.
-
CmPn/CmP Signaling Networks in the Maintenance of the Blood Vessel Barrier.J Pers Med. 2023 Apr 28;13(5):751. doi: 10.3390/jpm13050751. J Pers Med. 2023. PMID: 37240921 Free PMC article. Review.
-
Prefrontal cortex iron content in neurodegeneration and healthy subjects: A systematic review.Ibrain. 2025 Apr 10;11(2):215-227. doi: 10.1002/ibra.12195. eCollection 2025 Summer. Ibrain. 2025. PMID: 40546874 Free PMC article. Review.
-
The mechanism of ferroptosis and its related diseases.Mol Biomed. 2023 Oct 16;4(1):33. doi: 10.1186/s43556-023-00142-2. Mol Biomed. 2023. PMID: 37840106 Free PMC article. Review.
-
Targeting Iron Responsive Elements (IREs) of APP mRNA into Novel Therapeutics to Control the Translation of Amyloid-β Precursor Protein in Alzheimer's Disease.Pharmaceuticals (Basel). 2024 Dec 11;17(12):1669. doi: 10.3390/ph17121669. Pharmaceuticals (Basel). 2024. PMID: 39770511 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

