Computational Approaches for Acute Traumatic Brain Injury Image Recognition
- PMID: 35370919
- PMCID: PMC8964403
- DOI: 10.3389/fneur.2022.791816
Computational Approaches for Acute Traumatic Brain Injury Image Recognition
Abstract
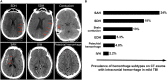
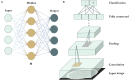
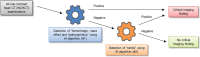
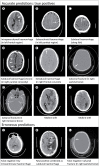
In recent years, there have been major advances in deep learning algorithms for image recognition in traumatic brain injury (TBI). Interest in this area has increased due to the potential for greater objectivity, reduced interpretation times and, ultimately, higher accuracy. Triage algorithms that can re-order radiological reading queues have been developed, using classification to prioritize exams with suspected critical findings. Localization models move a step further to capture more granular information such as the location and, in some cases, size and subtype, of intracranial hematomas that could aid in neurosurgical management decisions. In addition to the potential to improve the clinical management of TBI patients, the use of algorithms for the interpretation of medical images may play a transformative role in enabling the integration of medical images into precision medicine. Acute TBI is one practical example that can illustrate the application of deep learning to medical imaging. This review provides an overview of computational approaches that have been proposed for the detection and characterization of acute TBI imaging abnormalities, including intracranial hemorrhage, skull fractures, intracranial mass effect, and stroke.
Keywords: artificial intelligence; deep learning; evidence-based medicine; image recognition; precision medicine; traumatic brain injury.
Copyright © 2022 Lin and Yuh.
Conflict of interest statement
EY is an author of USPTO No. 62/269,778, Interpretation and quantification of emergency features on head computed tomography, and PCT Patent Application No. PCT/US2020/042811, Expert-level detection of acute intracranial hemorrhage on head CT scans, both assigned to Regents of the University of California. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Gentry LR, Godersky JC, Thompson B, Dunn VD. Prospective comparative study of intermediate-field MR and CT in the evaluation of closed head trauma. Am J Neuroradiol. (1988) 9:91–100. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Medical

