Perirenal Fat Thickness: A Surrogate Marker for Metabolic Syndrome in Chinese Newly Diagnosed Type 2 Diabetes
- PMID: 35370949
- PMCID: PMC8965868
- DOI: 10.3389/fendo.2022.850334
Perirenal Fat Thickness: A Surrogate Marker for Metabolic Syndrome in Chinese Newly Diagnosed Type 2 Diabetes
Abstract
Objective: Increasing evidence suggested that perirenal fat thickness (PrFT) was associated with metabolic risk factors. This study aimed to assess the association between PrFT and metabolic syndrome (MetS) in Chinese newly diagnosed type 2 diabetes (T2DM), further evaluating the ability of PrFT in identifying MetS.
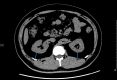
Method: A total of 445 Chinese newly diagnosed T2DM were enrolled in this study from January to June 2021. Demographic and anthropometric information were collected. PrFT was evaluated by CT scan on Revolution VCT 256. MetS was based on the Chinese Diabetes Society definition. Receiver operating characteristic (ROC) curve was conducted to assess the optimal cutoff value of PrFT in identifying MetS.
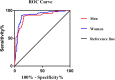
Results: Overall, the prevalence of MetS was 57.5% (95% CI: 54.0-64.0%) in men and 58.9% (95% CI: 52.3-65.5%) in women separately. The correlation analysis showed that PrFT was significantly correlated with metabolic risk factors like body mass index, waist circumference, triglycerides, high-density lipoprotein cholesterol, systolic blood pressure, diastolic blood pressure, uric acid, and insulin resistance. PrFT was also shown to be independently associated with MetS after adjustment for other confounders. The odds ratios (ORs, 95% CI) were 1.15 (1.03-1.38) in men and 1.31 (1.08-1.96) in women (P < 0.05). The ROC curves showed a good predictive value of PrFT for MetS. The areas under the curve of PrFT identifying MetS were 0.895 (95% CI: 0.852-0.939) in men and 0.910 (95% CI: 0.876-0.953) in women (P < 0.001). The optimal cutoff values of PrFT were 14.6 mm (sensitivity: 83.8%, specificity: 89.6%) for men and 13.1 mm (sensitivity: 87.6%, specificity: 91.1%) for women.
Conclusions: PrFT was significantly associated with MetS and showed a powerful predictive value for it, which suggested that PrFT can be an applicable surrogate marker for MetS in Chinese newly diagnosed T2DM.
Clinical trial registration: This study was registered in clinicaltrials.gov (ChiCTR2100052032).
Keywords: metabolic syndrome; newly diagnosed type 2 diabetes; optimal cut-off value; perirenal fat thickness; visceral adipose tissue.
Copyright © 2022 Guo, Tu, Chen and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Perirenal Fat Thickness is Associated with Metabolic Dysfunction-Associated Fatty Liver Disease in Type 2 Diabetes Mellitus.Diabetes Metab Syndr Obes. 2023 Jun 28;16:1953-1965. doi: 10.2147/DMSO.S415477. eCollection 2023. Diabetes Metab Syndr Obes. 2023. PMID: 37405319 Free PMC article.
-
Monocyte to High-Density lipoprotein and Apolipoprotein A1 Ratios: Novel Indicators for Metabolic Syndrome in Chinese Newly Diagnosed Type 2 Diabetes.Front Endocrinol (Lausanne). 2022 Jul 14;13:935776. doi: 10.3389/fendo.2022.935776. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35909551 Free PMC article.
-
Perirenal fat thickness as a superior obesity-related marker of subclinical carotid atherosclerosis in type 2 diabetes mellitus.Front Endocrinol (Lausanne). 2023 Oct 27;14:1276789. doi: 10.3389/fendo.2023.1276789. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37964956 Free PMC article.
-
Perirenal fat thickness contributes to the estimated 10-year risk of cardiovascular disease and atherosclerotic cardiovascular disease in type 2 diabetes mellitus.Front Endocrinol (Lausanne). 2024 Jul 8;15:1434333. doi: 10.3389/fendo.2024.1434333. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39040678 Free PMC article.
-
Biomarkers in metabolic syndrome.Adv Clin Chem. 2022;111:101-156. doi: 10.1016/bs.acc.2022.07.003. Epub 2022 Sep 6. Adv Clin Chem. 2022. PMID: 36427908 Review.
Cited by
-
The perirenal fat thickness was independently associated with serum uric acid level in patients with type 2 diabetes mellitus.BMC Endocr Disord. 2022 Aug 20;22(1):210. doi: 10.1186/s12902-022-01081-9. BMC Endocr Disord. 2022. PMID: 35987648 Free PMC article.
-
1H-NMR-based metabolomics reveals the preventive effect of Enteromorpha prolifera polysaccharides on diabetes in Zucker diabetic fatty rats.Food Sci Nutr. 2024 Mar 5;12(6):4049-4062. doi: 10.1002/fsn3.4061. eCollection 2024 Jun. Food Sci Nutr. 2024. PMID: 38873458 Free PMC article.
-
Associations of renal sinus fat with metabolic parameters, abdominal visceral adipose tissue, metabolic syndrome, fructose intake, and blood pressure control in obese individuals with hypertension: a cross-sectional study.J Nutr Sci. 2024 Dec 16;13:e94. doi: 10.1017/jns.2024.84. eCollection 2024. J Nutr Sci. 2024. PMID: 39703900 Free PMC article.
-
Opportunistic Detection of Chronic Kidney Disease Using CT-Based Measurements of Kidney Volume and Perirenal Fat.J Clin Med. 2025 Aug 20;14(16):5888. doi: 10.3390/jcm14165888. J Clin Med. 2025. PMID: 40869714 Free PMC article.
-
Perirenal Fat Thickness is Associated with Metabolic Dysfunction-Associated Fatty Liver Disease in Type 2 Diabetes Mellitus.Diabetes Metab Syndr Obes. 2023 Jun 28;16:1953-1965. doi: 10.2147/DMSO.S415477. eCollection 2023. Diabetes Metab Syndr Obes. 2023. PMID: 37405319 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

